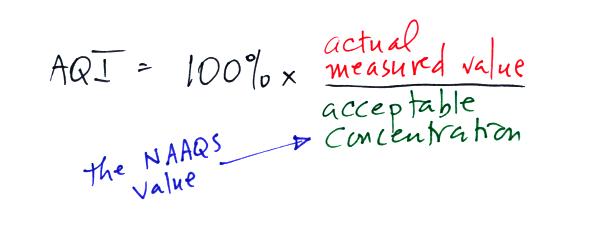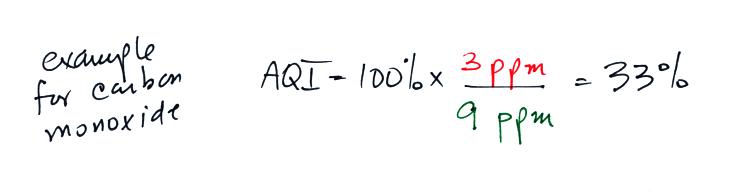Now we're ready to spend a couple of lectures on air
pollutants.
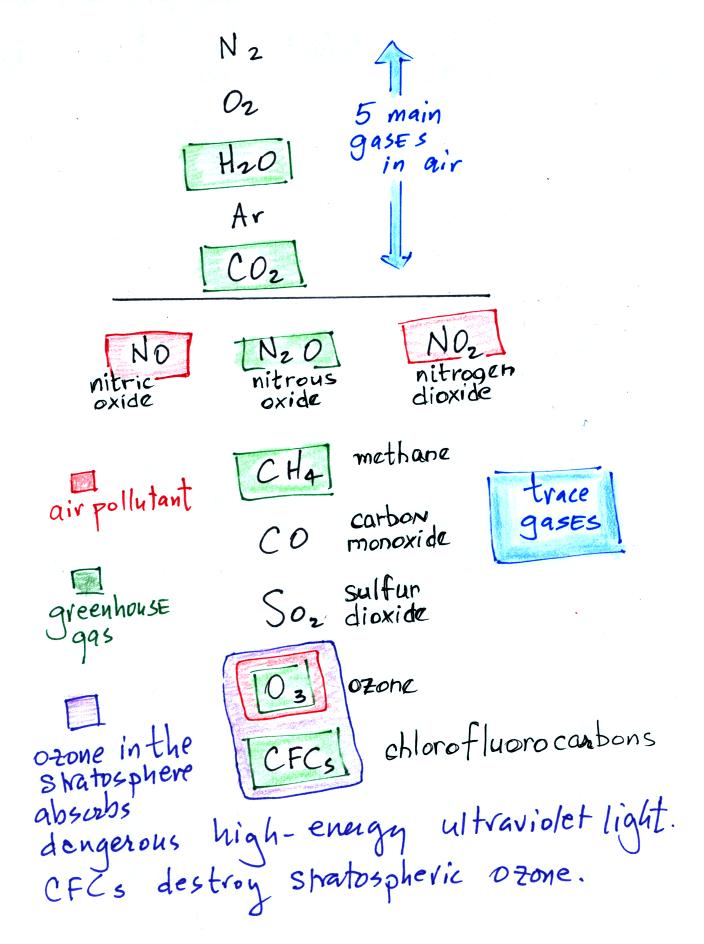
We listed
the 5 most abundant gases in the atmosphere below.
Several important trace gases have been added to the
list in
class. Trace gases are gases found in low
concentrations (and often the concentrations are variable).
Low concentrations doesn't mean they aren't
important, however.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing
gas),
chlorofluorocarbons,
and
ozone
are
all
greenhouse
gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic and learn more about how the
greenhouse effect actually works later in the course.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants.
Give ozone some special attention, it has sort of a Dr. Jeckyl and
Mr. Hyde personality
(i) Ozone in the
stratosphere (a layer of the atmosphere between about 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog (Los
Angeles-type smog).
(iii) Ozone is also a greenhouse gas.
In the next two lectures we'll learn about a few of the
main air pollutants.
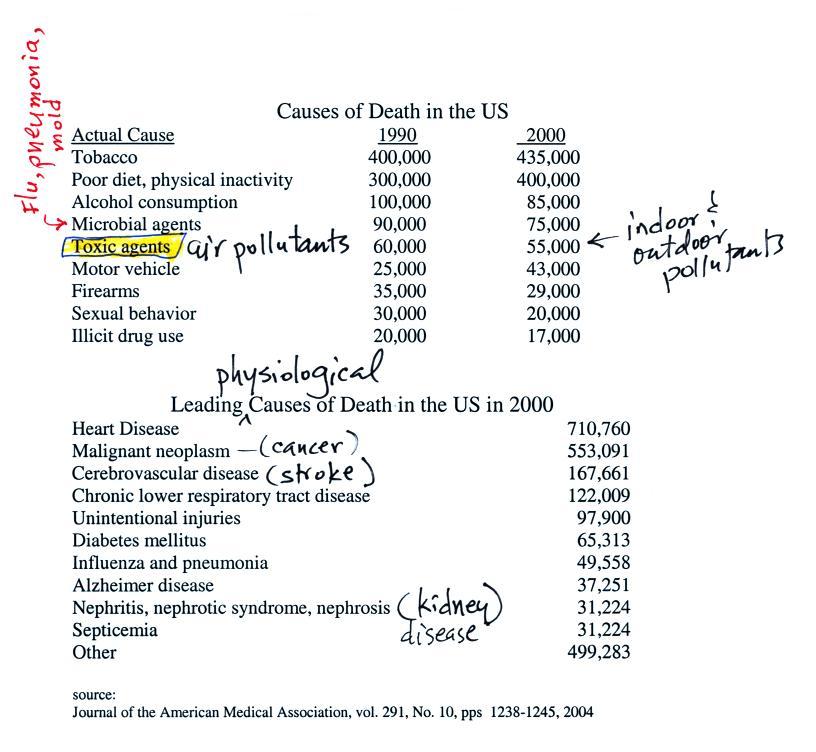
Air
Pollution is a serious health hazard in the US and around the
world (we'll mainly discuss outdoor pollution, but indoor air
pollution is also a problem).
Keep in mind that many of these
numbers are difficult to measure
and some may contain a great deal of uncertainty. The row that is
highlighted, toxic agents, contains estimates of deaths caused by
indoor and outdoor air pollution, water pollution, and exposure to
materials such as asbestos and lead both in the home and at the work
place. It is estimated that 60% of the deaths are due to exposure
to particulate matter, something that we will examine briefly in the
next
lecture.
Air pollution is a serious hazard
worldwide. Indoor air pollution is, in many places,
a more serious threat than outdoor air pollution.
The Blacksmith
Institute listed the Top 10 polluted places in the world in a
2007 report. The report has received a lot of worldwide
attention. If you go to this
address, you can view the report online or download and print a
copy of the report.
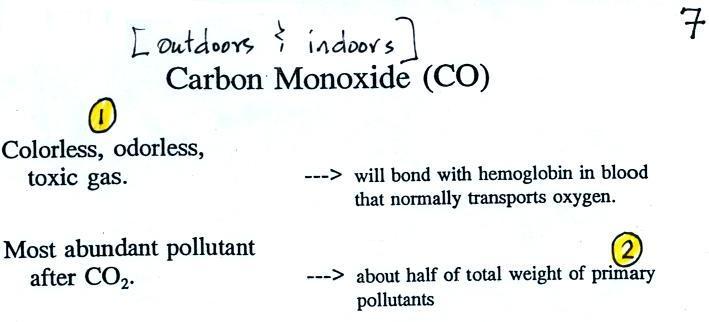
We'll start with carbon monoxide. Some basic
information is shown
below.
You'll find
additional information at the Pima
County Department of
Environmental Quality website and also at the US Environmental
Protection Agency
website.
We will mostly be talking about
carbon
monoxide found outdoors, where it would rarely reach fatal
concentrations. Indoors is a serious hazard indoors also where it
can (and does) build up to deadly concentrations. (
several people were almost killed in Tucson in December 2010)
Carbon monoxide is insidious, you can't smell it or see it
and it can kill you (Point 1).
Once
inhaled,
carbon
monoxide
molecules
bond
strongly
to
the
hemoglobin
molecules
in
blood
and
interfere
with
the
transport
of
oxygen
throughout
your
body.
The
article
above mentions that the CO poisoning victims
were put inside a hyperbaric (high pressure) chamber filled with pure
oxygen. This must force oxygen into the blood and displace the
carbon monoxide.
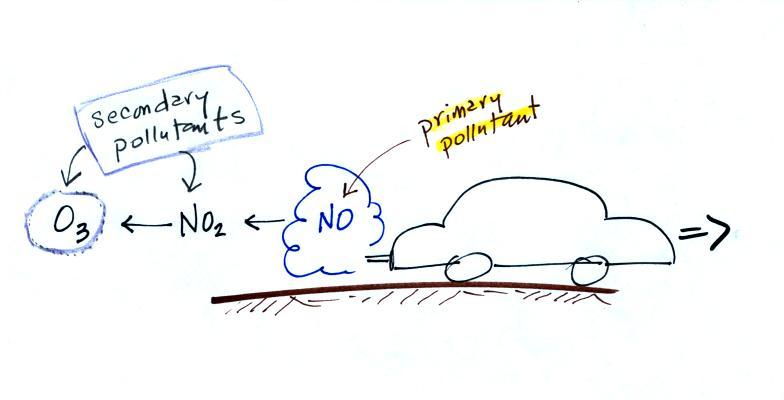
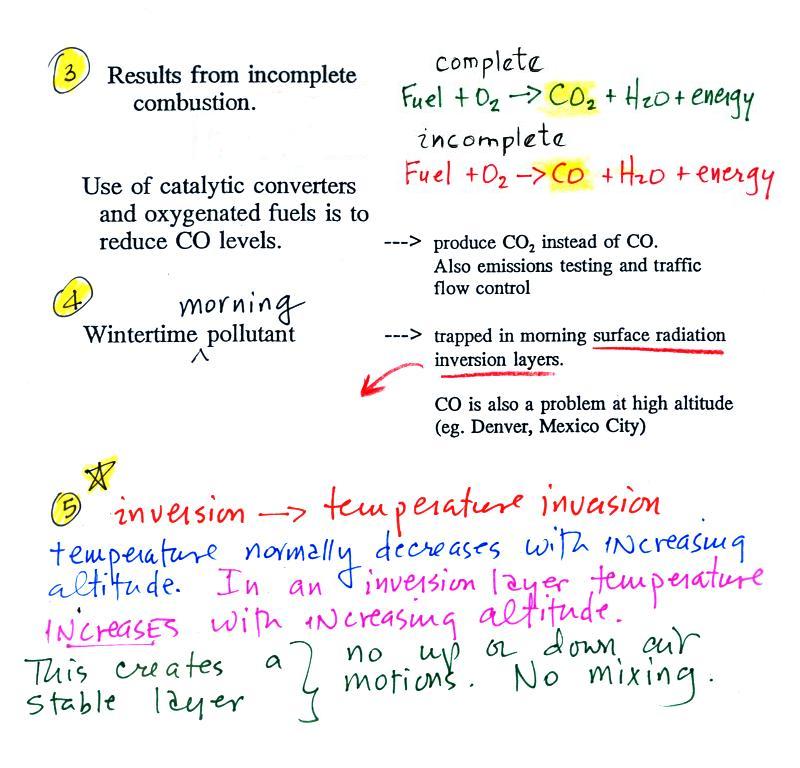
CO is a primary pollutant (Point 2
above). That means it goes
directly from a source into the air, CO is
emitted directly from an automobile tailpipe into the atmosphere for
example. The difference between
primary and secondary pollutants is probably explained
best in a series of pictures.
Nitric oxide, NO, and sulfur
dioxide, SO2, are also primary pollutants. Ozone is a
secondary pollutant (and here we are referring to tropospheric ozone,
not stratospheric ozone). It doesn't come directly from an
automobile tailpipe. It shows up in the atmosphere only
after a
primary pollutant has undergone a series of reactions.
Point 3
explains that CO is produced by incomplete
combustion of fossil
fuel (insufficient oxygen). Complete combustion would produce
carbon dioxide,
CO2. Cars and trucks
produce much of the CO in
the
atmosphere in Tucson.
Vehicles must now
be fitted with a catalytic
converter that will change CO into CO2 (and also NO into N2
and
O2
and hydrocarbons into H2O
and CO2).
In
large
metropolitain areas such as Pima County, vehicles
must
also
pass
an
emissions
test every
year and special formulations of gasoline (oxygenated fuels) are used
during the winter months to try to reduce CO emissions.
In the atmosphere CO concentrations peak on winter
mornings (Point 4).
Surface temperature inversion layers form on long winter nights when
the sky is clear and winds are calm. The ground cools quickly and
becomes colder than the air above. Air in contact with the
cold ground ends up colder than air above.
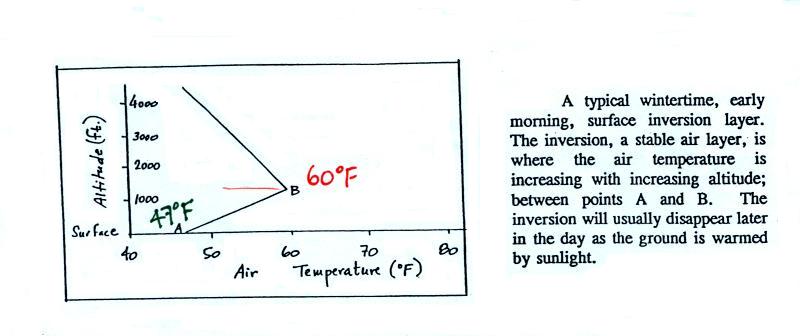
In a temperature inversion layer, air
temperature increases with increasing altitude (Point 5). This produces a
very stable layer of air
at ground
level (stable means the air is stagnant, there are no
up or down air motions). A very reasonable
wintertime morning
temperature profile in Tucson is
shown below:
Temperature increases from 47o
F at the
ground (Point A) to about 60o F at 1000 feet altitude (Point
B), that's the stable inversion layer. Temperature begins to
decrease with increasing altitude above Point B.
There is very little vertical
mixing in a stable air
layer.
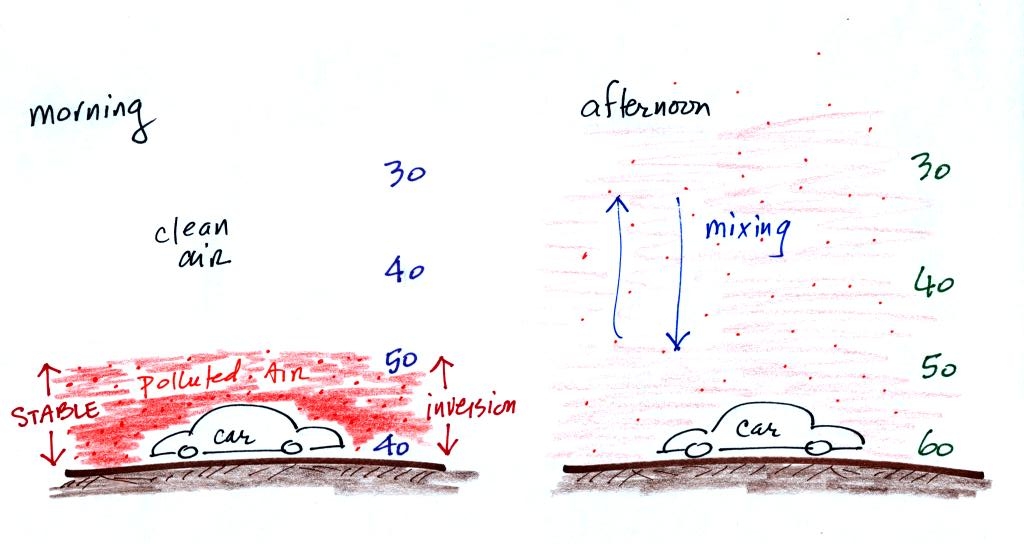
When CO is emitted into the thin
stable layer (left figure
above), the CO
remains in the layer and doesn't mix with cleaner air above. CO
concentrations build.
In the afternoon, the ground warms, the inversion layer
disappears, and the atmosphere becomes
more
unstable. CO emitted
into air at the surface mixes with cleaner air above. The CO
concentrations are effectively diluted.

Thunderstorms contain strong up
(updraft) and down (downdraft) air motions. Thunderstorms are a
sure indication of unstable
atmospheric conditions. When the
downdraft winds hit the ground they spread out horizontally.
These surface winds can sometimes reach 100 MPH, stronger than many
tornadoes.
The concentrations of several of the main pollutants are monitored in
large cities in the US and around the world. In Tucson,
carbon monoxide, ozone, and particulate matter are of primary concern
and daily measurements are reported in the city newspaper.
Let's imagine that the average carbon monoxide concentration in Tucson
air yesterday was 3 ppm. Is this a high and unhealthy value or
was the air quality OK? We need some more information. We
need to know what an acceptable concentration level for carbon monoxide
is. The EPA has done just that
The six main pollutants are listed above (there are many more).
The concentration of lead in air has decreased
significantly since lead was removed from gasoline (the
following quote is from a
Wikipedia
article
on
gasoline: "In the US,standards to phase out
leaded gasoline were first implemented in 1973 ..... In 1995, leaded
fuel accounted for only 0.6% of total gasoline sales ...... From 1
January 1996, the Clean Air Act banned the sale of leaded fuel
for use in on-road vehicles. Possession and use of leaded gasoline in a
regular on-road vehicle now carries a maximum $10,000 fine in the US.")
Rather
than report the actual measured values, an Air Quality Index value is
reported instead. The AQI is the ratio of the
measured to accepted
concentrations multiplied by 100%.
If we plug in the 3 ppm value mentioned above for carbon monoxide,
the AQI value would be
The air quality in this case would be good. Air becomes
unhealthy when the
AQI value exceeds 100%. The units "ppm", by the way,
stands for "parts per million." A CO concentration of 3 ppm would
mean that in 1 million air molecules 3 of them would be carbon monoxide.
Current
Air
Quality
Index
values
for
Tucson
are
available online.
So are we
have been talking about carbon monoxide found in
the atmosphere. Carbon monoxide is also a serious
hazard indoors where is can build to much higher levels than would ever
be found outdoors. You may remember having heard
about an incident at the beginning of the school year in 2007 where carbon
monoxide
from a malfunctioning hot water heater sickened 23 Virginia Tech
students in an apartment complex. The CO concentration is
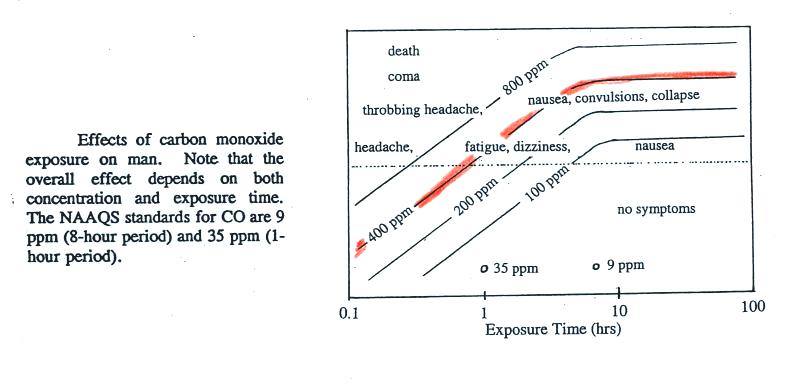
thought to have reached 500 ppm. You can get an idea of what
kinds of health effects concentrations this high could cause from the
figure below.
To get an idea of what effects 500 pm CO
concentrations could cause, we will follow the 400 ppm line (shaded
orange) from left to right.
At
exposure times less than 1 hour you should experience no
symptoms. Beginning at 1 hour you might experience headache,
fatique, and dizziness. Exposures of a few hours will produce
throbbing headache, nausea, convulsions, and collapse. The 400
ppm trace level approaches the level where CO would cause coma and
death. At Virginia Tech several students were found unconscious
and one or two had stopped breathing but they were revived.
Carbon monoxide
alarms are relatively inexpensive and readily available at any hardware
store. They will monitor CO concentrations indoors and warn you
when
concentrations reach hazardous levels. Indoors CO is
produced by gas furnaces and water heaters that are
either operating improperly or aren't being adequately vented
to the outdoors. A few hundred people are killed indoors by
carbon
monoxide every
year in the United States. You can learn
more about carbon monoxide hazards and risk prevention at the Consumer Product
Safety Commission web page.
Before discussing tropospheric ozone, which is
a pollutant, a quick reminder that there are both "good" and "bad"
types of ozone.
Ozone has a Dr.
Jekyll
and Mr. Hyde personality. Ozone
in
the stratosphere (the ozone layer) is beneficial, it absorbs dangerous
high
energy ultraviolet light (which would otherwise reach the ground and
cause skin cancer, cataracts, and many other problems). It is
also easy to make ozone in the stratosphere; molecular oxygen and
ultraviolet light are all that are required.
Ozone in the troposphere is bad, it is a
pollutant.
That is the stuff we will first be concerned with here.
Tropospheric
ozone is a key component of photochemical smog (also known as Los
Angeles-type smog). Making ozone in the troposphere is also a
more complex process as we will see below. We'll
make some photochemical smog as a classroom emonstration. This
will require ozone (and a hydrocarbon of some
kind). We'll use the simple stratospheric recipe for making
ozone in the demonstration rather than the more complex tropospheric
process..
At the top of this confusing figure you see that a
more complex
series
of
reactions is responsible for the production of tropospheric
ozone. The production of tropospheric
ozone begins with nitric
oxide
(NO). NO is produced when nitrogen and oxygen in air are heated
(in an
automobile engine for example) and react. The NO can then react
with oxygen to make nitrogen dioxide,
a
poisonous
brown-colored
gas..
Sunlight
can dissociate (split)
the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2
react in a 4th step to make ozone (O3).
Because
ozone
does
not come directly from an automobile tailpipe or factory chimney,
but only shows up after a series of reactions, it is a secondary
pollutant. Nitric oxide would be the primary pollutant in
this example.
NO is produced early in the day (during the morning rush
hour).
The concentration of NO2
peaks
somewhat later. Peak ozone concentrations are usually found in
the afternoon. Ozone concentrations are also usually higher in
the summer than in the winter. This is because sunlight plays a
role in ozone production and summer sunlight is more intense than
winter sunlight.
As shown in the figure below,
invisible ozone can react with a hydrocarbon of some kind which is also
invisible to make a
product
gas. This product gas sometimes condenses to make a visible smog
cloud or haze. The cloud is composed of very small droplets or
solid particles. They're too small to be seen but they are able
to scatter light - that's why you can see the cloud.
The class demonstration of
photochemical smog is summarized
below. We begin by using the UV
lamp to create and fill the flask with
ozone. Then a few pieces of fresh lemon peel were added to the
flask. A whitish cloud quickly became visible (the cloud is
colored brown in
the figure below).