Tuesday Apr. 3, 2012
click here to
download today's notes in a more printer friendly format
"Church music" this week leading up to Easter. We started
with "Down
by the Riverside" and a
cool
verion
of
"Amazing
Grace" from The
Blind Boys of Alabama. Here are a few bonus
selections: "Satisfied
Mind",
and
"Way
Down
in
the
Hole".
All of the various Optional Assignments (except for the Controls
of Temperature assignment) that you've been turning in have been graded
and were returned in class today. Click here and you'll find
links to answers for each of the assignments.
If you turned in a "Fog in Tucson" 1S1P report at the start
of class
today, you have earned a GreenCard. The GreenCards will be
distributed before the quiz on Thursday. You can still turn in
the report or the Stability worksheet for 1S1P pts (both are due by
next Tuesday).
The last
big topic we will cover
before next week's quiz is precipitation formation and types of
precipitation. Only two of the 10 main cloud types (nimbostratus
and cumulonimbus) are able to produce
significant amounts of
precipitation. Why is that?
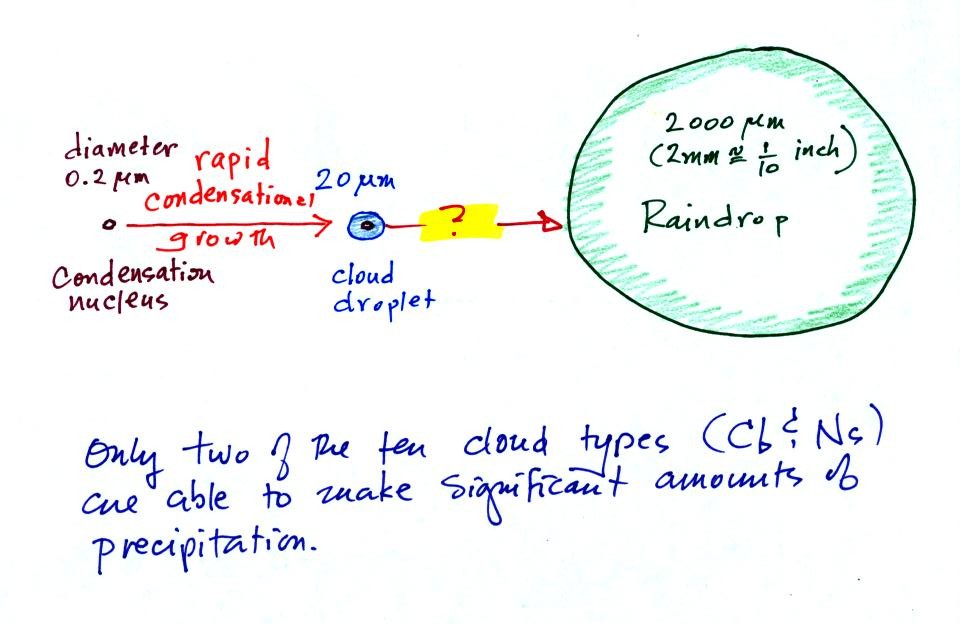
This figure shows typical sizes of
cloud
condensation nuclei (CCN), cloud droplets, and raindrops (a human hair
is about 50 μm thick for comparison). As
we
saw in the cloud in a bottle demonstration it is relatively easy to
make cloud droplets. You cool moist air to the dew point and
raise the RH to 100%. Water vapor
condenses pretty much instantaneously onto a cloud condensation nucleus
to form a cloud droplet. It
would take much longer (a day or more) for condensation to turn a cloud
droplet
into a
raindrop. You know from personal experience that once a cloud
forms you don't have to wait that long for precipitation to begin to
fall.
Part of the problem is that it
takes quite a few 20 μm
diameter cloud
droplets to make one 2000 μm diameter
raindrop.
Here's a similar question, but one that you might be able to look
at and answer.
It would take 64 individual sugar
cubes to make a 4 cube x 4 cube x 4 cube cube. That is because
the bigger cube is 4 times wider, 4 times deeper, and 4 times
taller. Volume is the product of all three dimensions. (27
sugar cubes would be
needed to make a 3 x 3 x 3 box etc)
The raindrop is 100 times wider,
100 times
deeper, and 100 times taller than the cloud droplet. The raindrop
has a volume that is 100 x 100 x 100 = 1,000,000 (one million) times
larger than the volume of
the cloud droplets. It takes about a million cloud
droplets to make one average size raindrop.
Fortunately
there
are
two
processes
capable
of quickly
turning small cloud droplets
into much larger precipitation particles in a cloud.

The collision coalescence process
works in clouds that
are
composed of water droplets only. Clouds like this are only found
in
the tropics. We'll see that this is a pretty easy process to
understand.
This process will only produce
rain, drizzle, and
something called virga (rain that evaporates before reaching the
ground).
The ice crystal process produces precipitation everywhere
else.
This is the process that makes rain in
Tucson, even on the hottest day in the summer (summer thunderstorm
clouds are tall and reach into cold parts of the atmosphere, well below
freezing. Hail and graupel often
fall from these storms; proof that the precipitation started out as an
ice particle). There is one part
of this process that is a little harder to understand.
This
process can produce a variety of different kinds of precipitation
particles (rain, snow, hail, sleet, graupel, etc).
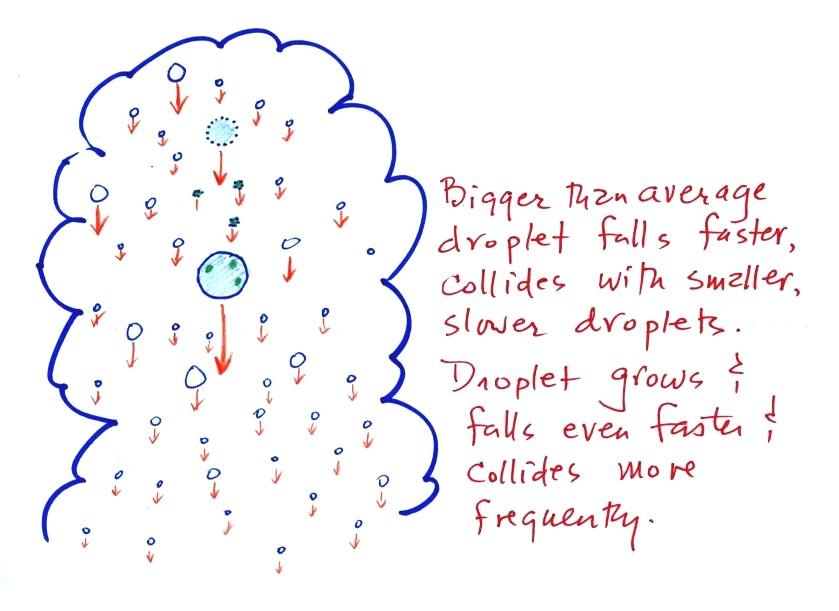
The collision coalescence process
works in a cloud
filled with cloud droplets of different sizes. The larger
droplets fall
faster than the small droplets. A larger-than-average cloud
droplet will overtake and collide with smaller slower moving
ones.
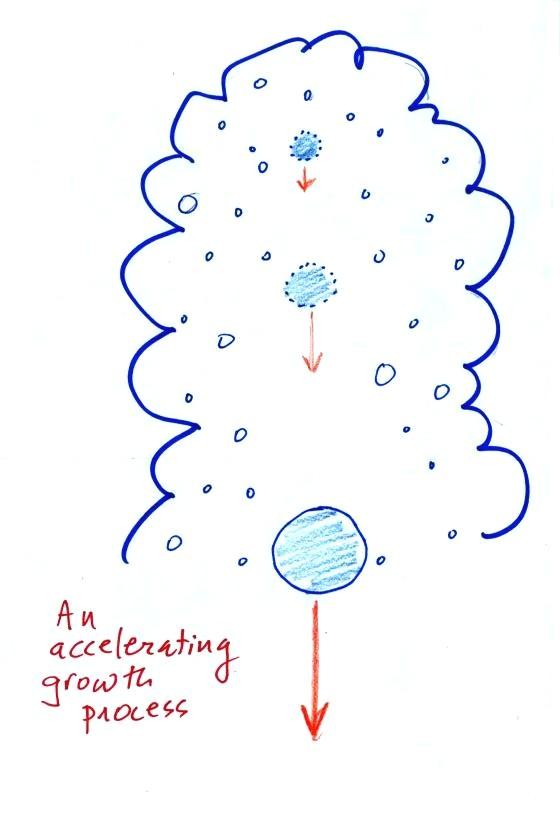
This is an accelerating growth
process.
The
falling droplet
gets
wider, falls faster, and sweeps out an increasingly larger volume
inside the cloud. The bigger the droplet gets the faster it
starts to grow (think of a growing ball of snow as it rolls down a
snow-covered hill and picks up snow, grows, and starts to roll faster
and faster; or think of an avalanche that
gets bigger and moves faster as it travels downslope)
A larger than average cloud droplet can very quickly grow to raindrop
size.
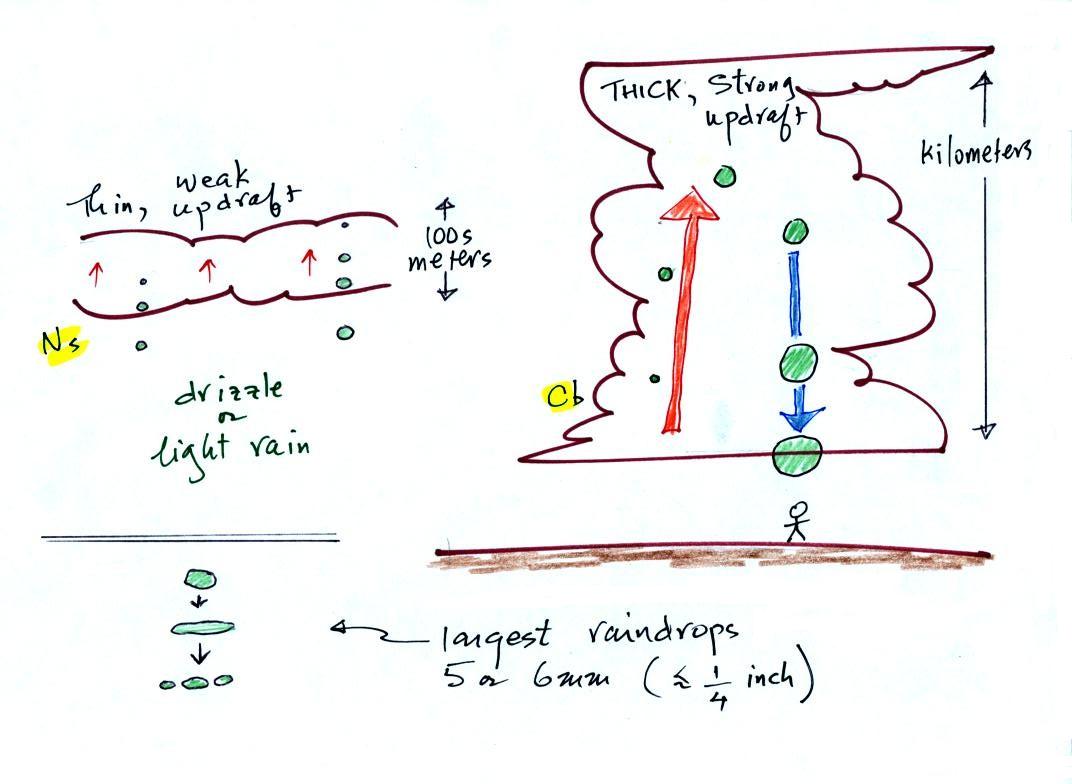
The figure shows the two
precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns clouds
are thinner
and have weaker updrafts than Cb clouds. The largest raindrops
fall from Cb clouds because the droplets spend more time in the cloud
growing. In a Cb cloud raindrops can grow while being carried upward by
the updraft and also when falling in the downdraft.
Raindrops grow up to about 1/4 inch in diameter.
When
drops get
larger than that, wind resistance flattens out the drop as it falls
toward the ground. The drop begins to "flop" around and breaks
apart
into several smaller droplets. Solid precipitation particles such
as hail can get much larger (an inch or two or three in diameter).
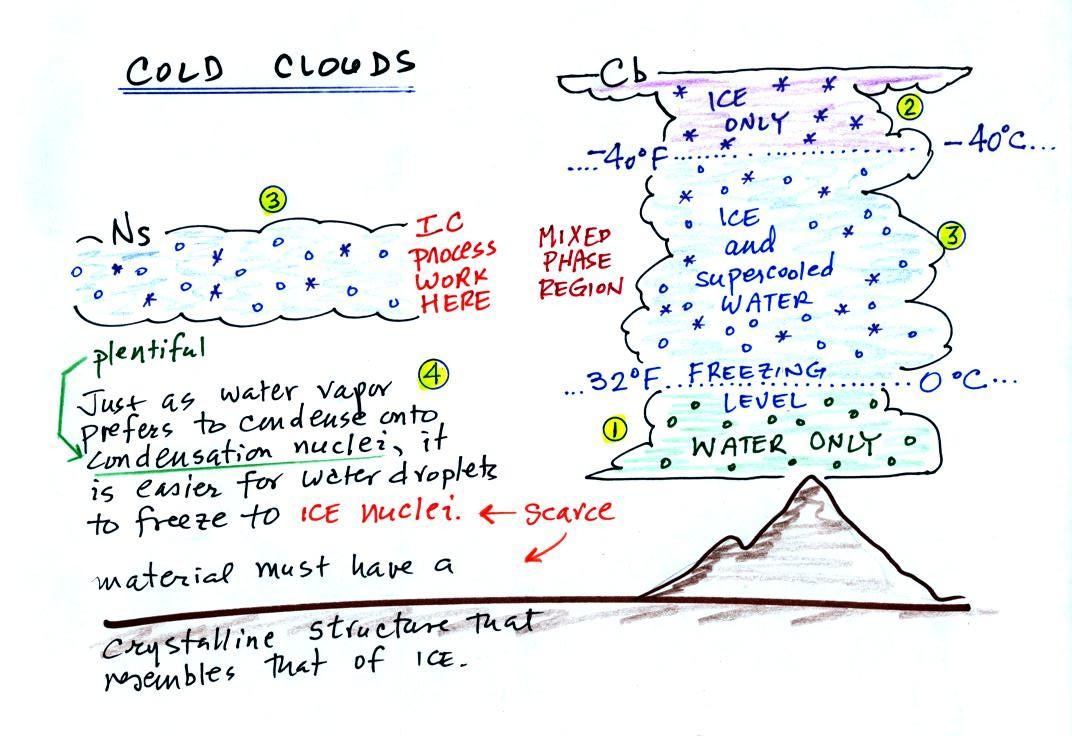
The ice crystal process works in
cold clouds, clouds that
contain ice crystals.
The bottom of the thunderstorm,
Point 1, is warm
enough
(warmer than freezing) to just
contain water
droplets. The top of the thunderstorm, Point 2, is colder than
-40 F (which, coincidentally, is equal to -40 C) and just contains ice
crystals. The
interesting part of the
thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains both
supercooled water
droplets (water that has
been cooled to below freezing but hasn't frozen) and ice
crystals.
This is called the mixed phase
region. This is where the ice crystal process will be able
to produce
precipitation. This is also where the electrical charge that
results in lightning is created.
The supercooled water droplets aren't able to freeze even though
they
have been cooled below freezing. At Point 4 we see this is
because it is much
easier for small droplets of water to freeze onto an ice crystal
nucleus or for water vapor to be deposited onto an ice crystal nucleus
(just like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small droplet
of pure water). Not just any material will work as an ice nucleus
however. The material must have
a crystalline structure that is like that of ice. There just
aren't very many materials with this property and as a result ice
crystal nuclei are rather scarce.
Here are a couple of demonstrations involving
supercooled water that I didn't have time to show in class. In the first
demonstration, some supercooled water (cooled to -6 F (-21 C)) is
poured into a glass bowl sitting at room temperature. Just
pouring the water into the bowl is enough of a "disturbance" to cause
the supercooled water to freeze. Just bumping a bottle of
supercooled water in
the second video is enough to cause the water to freeze.
We'll see
next how the ice crystal process works, it's a 3-step process.
This is the "tricky" part of the ice crystal process.
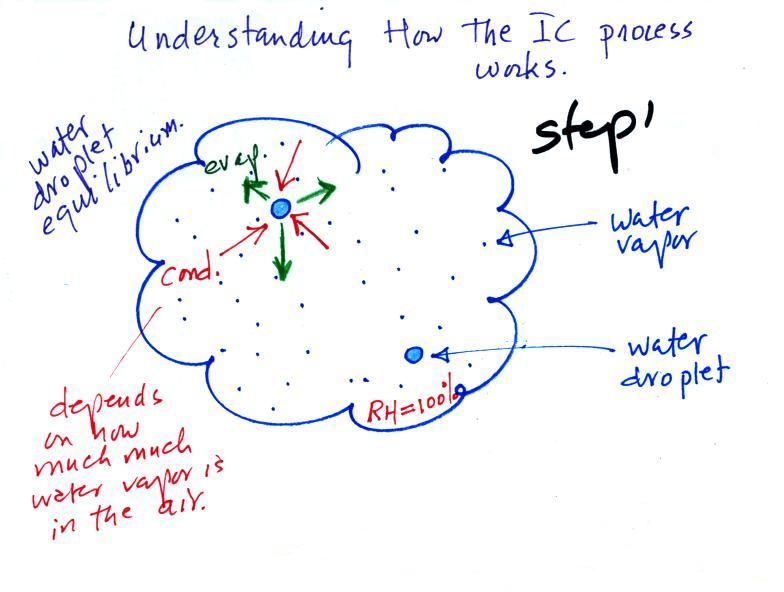
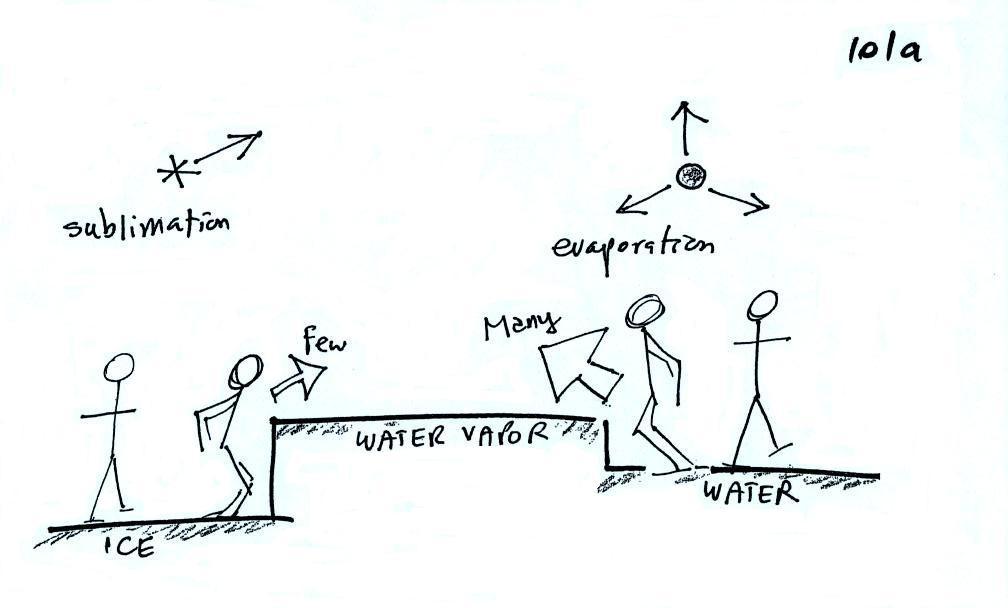
The first figure above (see p.101
in the photocopied
Class
Notes)
shows a water droplet in equilibrium with its surroundings. The
droplet
is evaporating (the 3 green arrows in the figure). The rate of
evaporation will depend on the temperature of the water droplet.
The droplet is surrounded by air that is saturated with water vapor
(the droplet is inside a cloud where the relative humidity is
100%). This means there is enough water vapor to be able to
supply 3 arrows of condensation. Because the droplet loses and
gains water vapor at equal rates it doesn't grow or shrink.
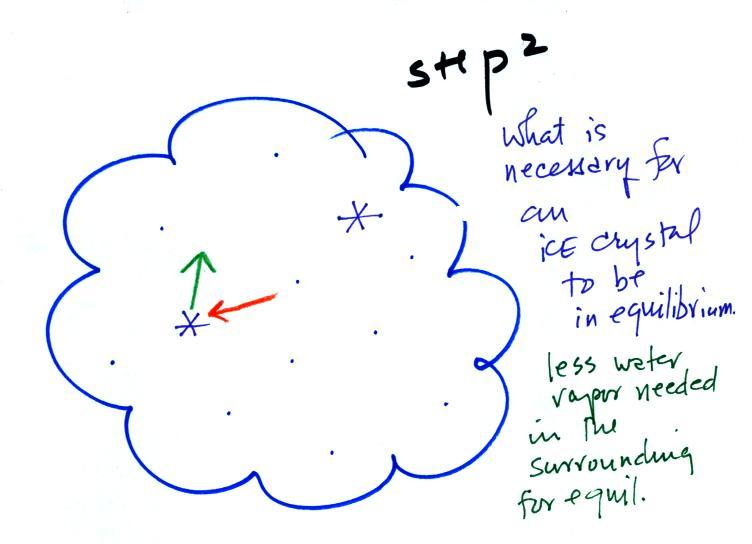
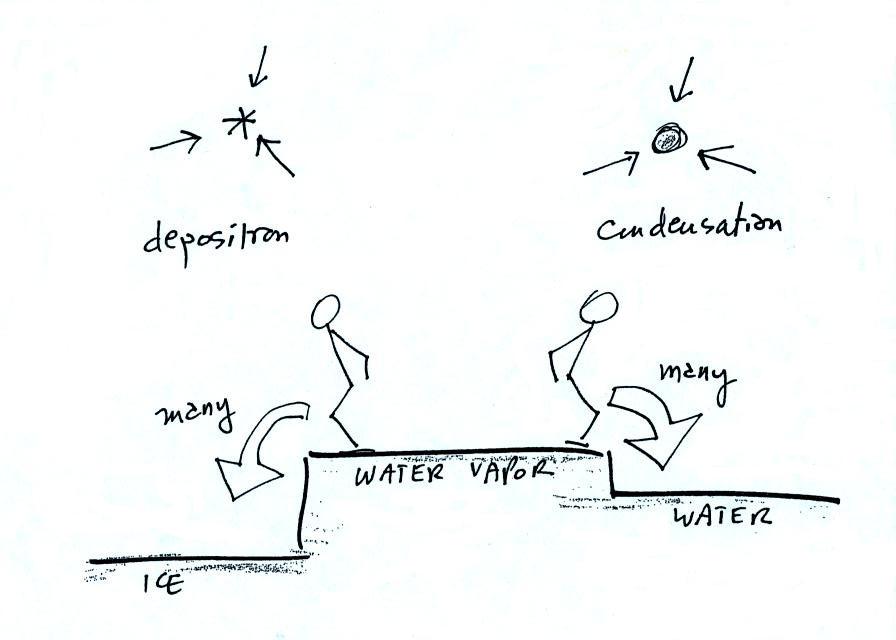
This figure shows what is required
for an ice crystal (at
the same
temperature) to be in equilibrium with its surroundings. First,
the ice crystal won't evaporate as rapidly as the water droplet (only 1
arrow is shown). Going from ice to water vapor is a bigger
"jump" than going from water to water vapor. There won't be as
many
ice molecules with enough energy to make that jump. A sort of
analogous situation is shown in the figure below. The class
instructor could and most of the people in the room could jump from the
floor to the top of a 12 or 15 inch tall box. It would be much
tougher to jump to the top of the table (maybe 30 inches off the
ground) or the podium (maybe 36 inches). There wouldn't be as
many people able to do that. Guess what I might be trying this
weekend in my
backyard.
To be in equilibrium the ice crystal only needs 1 arrow of
condensation. There doesn't need to be as much water vapor in the
air surrounding the
ice crystal to supply this lower rate of condensation. We could
have drawn in 2 arrows also, just so long as it isn't 3.
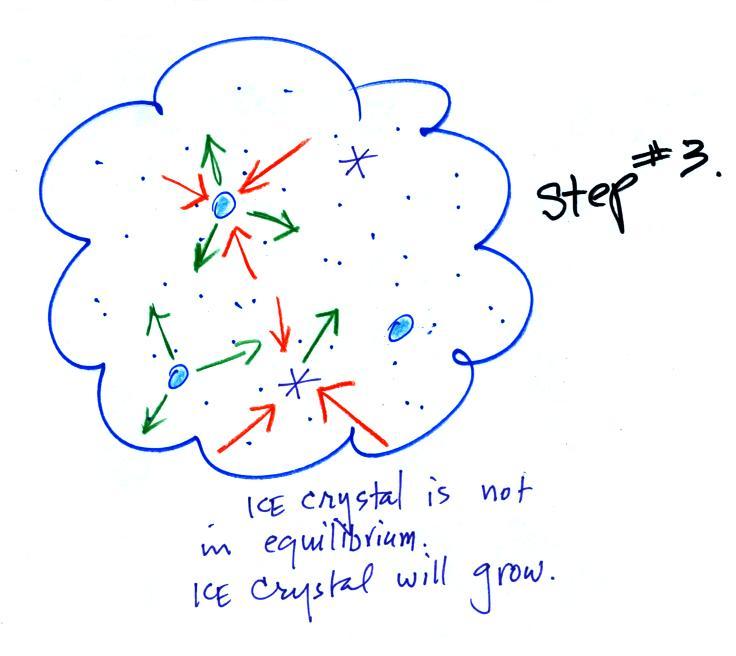
Now what happens in the mixed phase region of a cold cloud
is that
ice crystals find themselves in the very moist surroundings needed for
water droplet equilibrium. This is shown below.
The water droplet is in equilibrium
(3 arrows of evaporation
and 3
arrows of condensation) with the surroundings. The ice crystal is
evaporating more slowly than the water droplet. Because the ice
crystal is in the same surroundings as the water droplet water vapor
will be condensing onto the ice crystal at the same rate as onto the
water droplet. The ice
crystal isn't in equilibrium, condensation
(3 arrows) exceeds evaporation (1 arrow) and the ice crystal will
grow. That's
what makes the ice crystal process work.
The equal rates of condensation are shown in the figure
below using the
earlier analogy.
Most everyone can manage to make the big or the small jump down.
Now
we
will
see
what can happen once the ice crystal has had a chance to
grow a little bit.
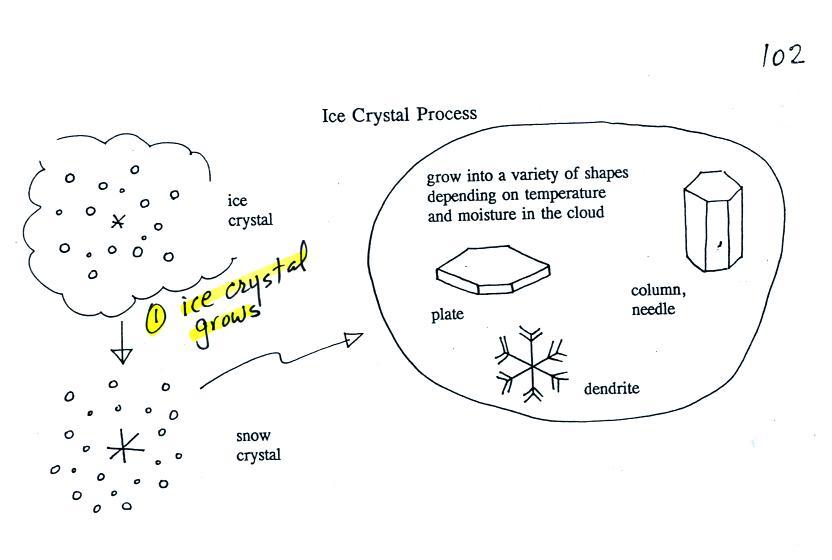
Once an ice
crystal has grown a
little bit it becomes a snow crystal (this figure is on p. 102 in the
photocopied classnotes). Snow crystals can have a variety of
shapes
(plates, dendrites, columns, needles, etc.; these are called crystal
habits) depending on the conditions (temperature and
moisture)
in the cloud. Dendrites are the most common because they form
where there
is the most moisture available for growth. With more raw material
available it makes sense there would be more of this particular snow
crystal
shape.
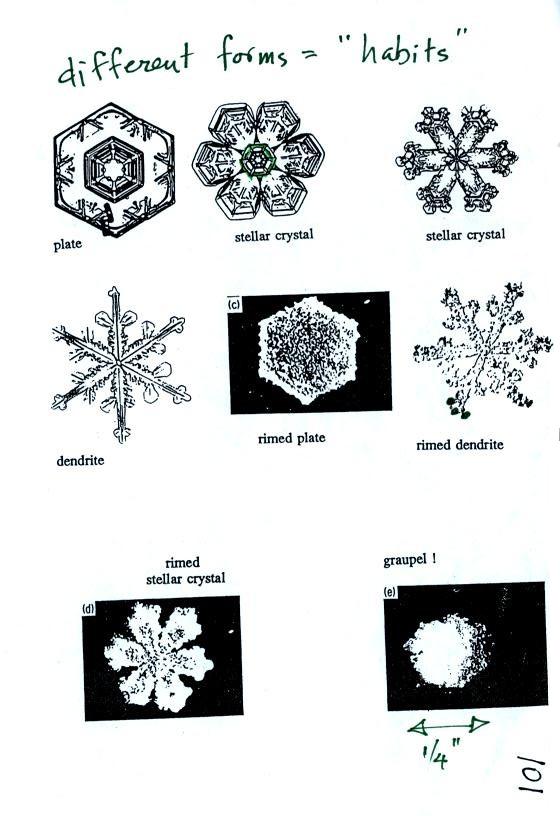
Here
are some actual photographs of snow crystals (taken with a
microscope). Snow crystals are usually 100 or a few 100s of
micrometers
in diameter (tenths of a millimeter in diameter). The different
shapes are called "habits".
You'll
find some much better photographs and a pile of addtional
information
about snow crystals at www.snowcrystals.com.
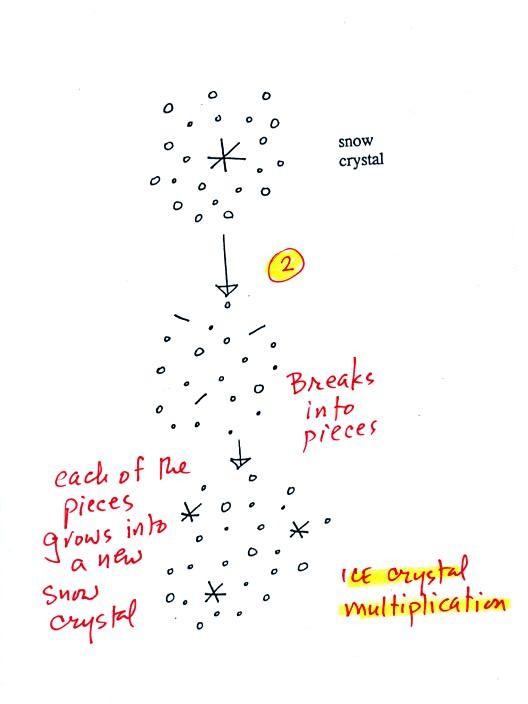
A
variety of things can happen once a snow crystal forms. First it
can
break into pieces, then each of the pieces can grow into a new snow
crystal. Because snow crystals are otherwise in rather short
supply, ice
crystal multiplication is a way of increasing the amount of
precipitation that
ultimately falls from the cloud.
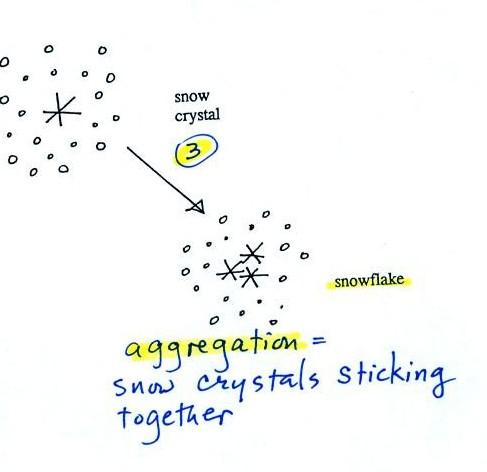
Several snow
crystals can collide
and stick together to form a snowflake. Snow crystals are small,
a few
tenths of a millimeter across. Snowflakes can be much larger and
are made
up of many snow crystals stuck together. The sticking together or
clumping together of snow crystals is called aggregation (I frequently
forget this term and don't expect you to remember it either)

Snow crystals can
collide with supercooled water droplets. The
water
droplets may stick and freeze to the snow crystal. This process
is called
riming or accretion (note this isn't called collision coalescence even
though
it is the same idea). If a snow crystal collides with enough
water
droplets it
can be completely covered with ice. The resulting particle is
called
graupel. Graupel is sometimes mistaken for hail
and is
called soft hail or snow pellets. Rime ice has a frosty milky
white
appearance. A graupel particle resembles a miniature snow
ball. Or smaller finer grained version of the shaved ice in a "snow cone."
Graupel
particles
often
serve
as
the
nucleus
for
a
hailstone.
Riming and graupel are terms you should remember.
This figure gives you an idea of
how hail forms.
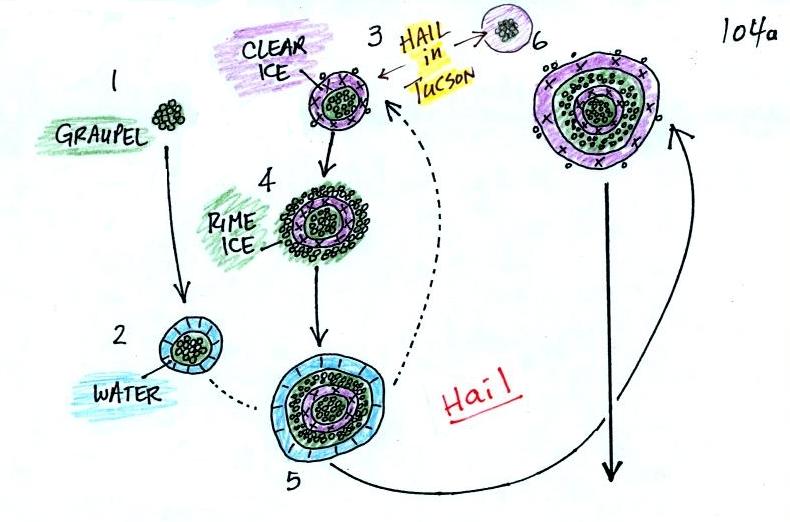
In the
figure
above a hailstone
starts with a graupel particle (Pt. 1, colored green to represent rime
ice). The
graupel falls or gets carried into a part of the cloud where it
collides with a
large number of supercooled water droplets which stick to the graupel
but don't
immediately freeze. The graupel gets coated with a layer of water
(blue) at Pt. 2. The particle then moves into a colder part of
the cloud
and the
water layer freeze producing a layer of clear ice (the clear ice,
colored
violet, has a distinctly different appearance from the milky white rime
ice), Pt. 3. In Tucson this is often the only example of hail that you
will see:
a graupel particle core with a single layer of clear ice.
Hail that falls to the ground in Tucson usually just has a graupel core
and a single layer of clear ice. In the severe thunderstorms in
the Central Plains, the hailstone can
pick up additional layers of rime ice and clear ice and hailstones can
be composed
of many
alternating layers of rime and clear ice. An unusually
large
hailstone (around 3 inches in diameter) has been cut in half to show
(below)
the different layers of ice. The picture below is close to actual
size. If something like this were to hit you in the head it would
split your skull open. Here's some pretty good video of a hailstorm in Phoenix.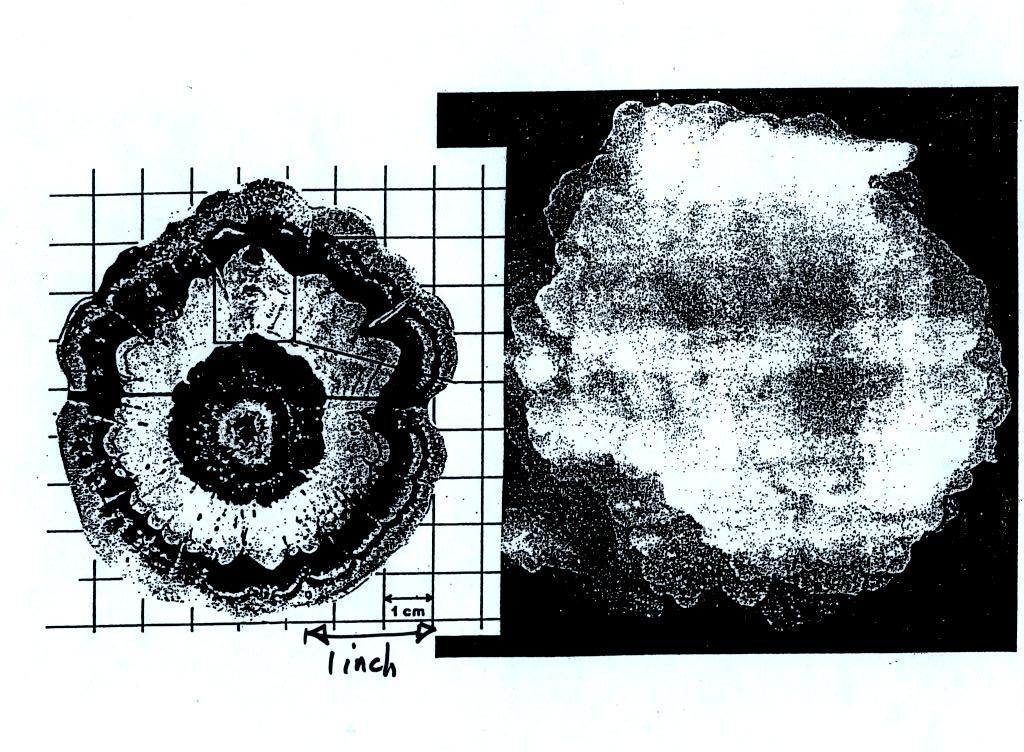
Hail is produced
in strong
thunderstorms with tilted updrafts. You would never see hail
(or graupel) falling from a nimbostratus cloud. A new record was
apparently set for a large
hailstone in Hawaii in March of this year. Hawaii is an
unusual place for hail this large to be found.
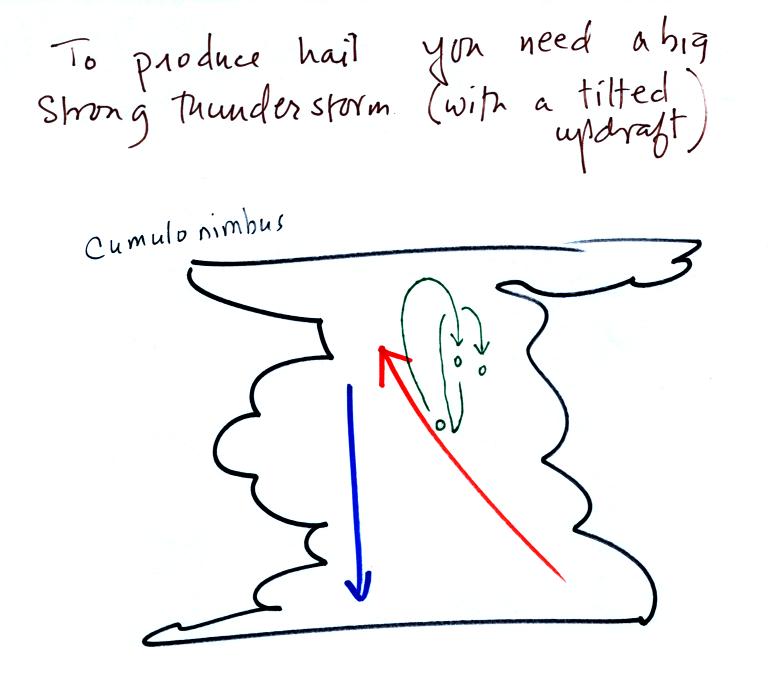
This figure wasn't
shown in class. The growing
hailstone can fall
back into the updraft (rather than falling out of the cloud) and be
carried
back up toward the top of the cloud. In this way the hailstone
can
complete several cycles through the interior of the cloud. The
article above mentions a supercell thunderstorm. We will discuss
these later in the semester.
Finally on p. 103 in the ClassNotes
are illustrations of some of the
things that can happen once a
precipitation particle falls from a cloud. I've split this into
two groups for clarity.

Essentially all the rain that falls
in Tucson is produced by the ice crystal process. The left figure
above shows how this happens. A falling graupel particle or a
snow
flake moves into warmer air and melts. The resulting drops of
water fall the rest of the way to the ground and would be called
RAIN.
In the middle picture graupel particles can survive the trip to
the ground without melting even in the summer. Many people on the
ground would call this hail but that wouldn't be quite right.
Graupel is less common in the winter because it comes from
thunderstorms and they don't form very often in the winter. Snow
can survive the trip to the ground in the winter but not the summer.
Sometimes the falling raindrops will evaporate before reaching the
ground. This is called VIRGA and is pretty common early in the
summer thunderstorm season in Arizona when the air is still pretty
dry.
Lightning that comes from thunderstorms that aren't producing much
precipitation is called "dry lightning" and often starts brush fires.
Rain will sometimes freeze before reaching the ground. The
resulting particle of clear ice is called SLEET. FREEZING RAIN by
contrast only freezes once it reaches the ground. Everything
on
the
ground (the image shows a car) can get coated with a thick
layer of ice. It is
nearly impossible to
drive
during one of these "ice storms." Sometimes the coating of ice
is heavy enough that branches
on
trees
are
broken and power
lines
are
brought
down. It sometimes takes several days for power to be
restored.