| Semester |
MWF class |
T Th class |
| S12 |
61% |
63% |
| S11 |
62% |
--- |
| S10 |
62% |
61% |
| S09 |
60% |
--- |
| S08 |
64% |
66% |
I would suggest you download the Practice Quiz if you didn't take it so that you can become familiar with the quiz format. You can check your answers against the answers that are available online. Quiz #1 in just over a week will cover Practice Quiz material plus new material that we cover between now and then.
The Experiment #1 reports were collected today. It generally takes a week, maybe a little more, to get them graded. Meanwhile the Experiment #2 materials should be distributed on Thursday. If you haven't returned your Experiment #1 materials please do so as soon as you can so that they can be cleaned up and handed out to the Expt. #2 people. The 1S1P Assignment #1 reports were also collected today.
A take-home Optional Assignment was handed out in class; the assignment is due on or before the start of class next Tuesday (Feb. 14). Be sure to have the assignment done before you come to class.
We started working on the three steps needed to understand why warm air rises and cold air sinks before the Practice Quiz last Thursday. The first step was learning about the ideal gas law. We'll finish all of that up today.
First though a couple of examples involving the ideal gas law, just to refresh your memory.

A can of spray paint is a sealed
rigid container. That means N (number of gas molecules) and V
(volume) stay constant. Heating up a can of spray paint
(something you shouldn't do) will increase the temperature and
increase the pressure. If the pressure gets too high the can will
explode.
In this next example we add some air to a tire.
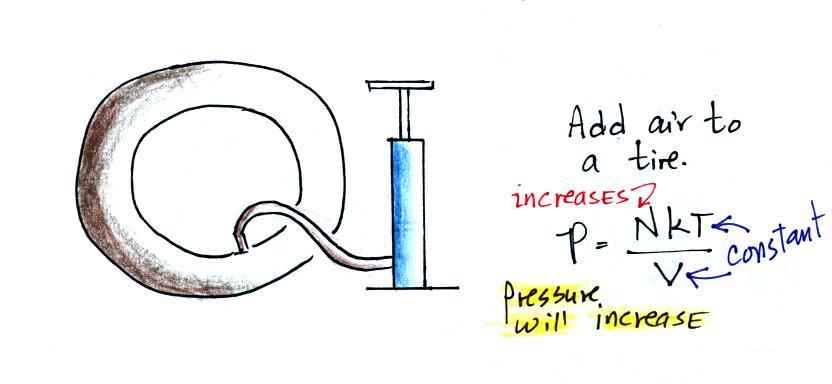
In this case the volume, V, and the temperature of the air, T,
remain constant. But we are adding air so N will increase.
This causes pressure to increase.
A volume of air in the atmosphere will behave somewhat differently. The volume can change. The thing that will cause the volume to change is creating an imbalance between the two pressures (two forces) shown in the figure below.
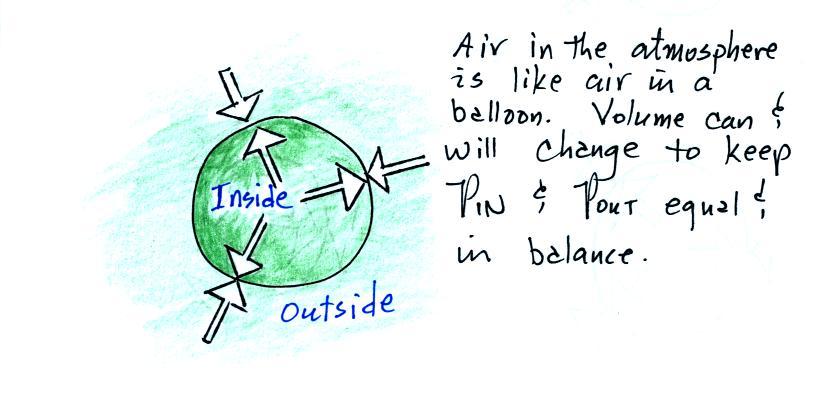
In this next example we add some air to a tire.

A volume of air in the atmosphere will behave somewhat differently. The volume can change. The thing that will cause the volume to change is creating an imbalance between the two pressures (two forces) shown in the figure below.

Here the pressure of the air
outside the volume that is pushing inward and trying to crush the
balloon is balanced by an outward pointing pressure of equal intensity
from the air inside the parcel [a parcel is just a volume of
air].
If we change something inside the balloon and upset this balance, the balloon will expand or shrink in an effort to bring the inward and outward pointing forces back into balance.

Charles' Law is a special situation involving the ideal gas
law. We can change conditions inside the balloon but will require
that the pressure of the air inside the parcel remain constant (P
inside is always trying to stay equal to P outside).
We'll look specifically at what happens if we heat or cool inside a parcel. This is a little more detailed version than was given in class.
First let's imagine warming the air inside a balloon.
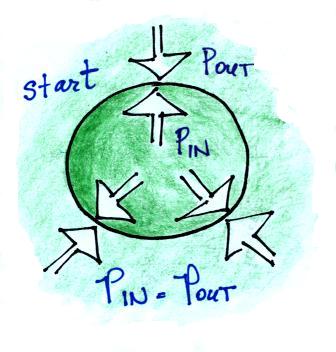
We'll start with air inside the balloon that is exactly the same
as the air outside the balloon (same air density, temperature, and
pressure inside and outside the balloon).
Next we'll warm the air inside the balloon but leave the air outside alone.
Increasing the temperature will momentarily increase the pressure. This creates an imbalance, so this is a temporary situation. Now that P inside is greater than P outside the balloon will expand.
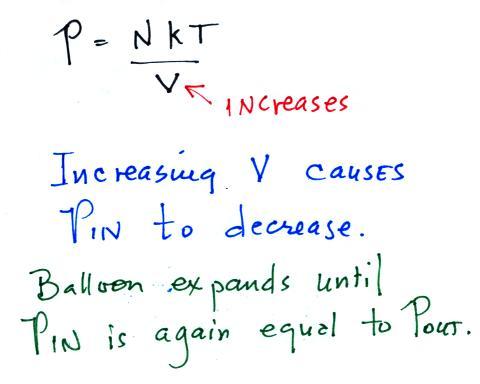
Increasing V in the ideal gas law equation will decrease P.
The balloon will keep expanding until P inside is back in
balance
with P outside.
We're left with a balloon that is larger, warmer, and filled with lower density air than it was originally.
The pressures inside and outside are again the same. The pressure inside is again equal to its starting value. The 1st form of the ideal gas law show you that you can increase the temperature and volume of a parcel together in a way that keeps pressure constant (which is what Charles' law requires). The 2nd version of the equation shows that by increasing the temperature and decreasing the density together you can keep pressure constant.
We can go through the same kind of reasoning and see what happens if we cool the air in a parcel.

If we change something inside the balloon and upset this balance, the balloon will expand or shrink in an effort to bring the inward and outward pointing forces back into balance.

We'll look specifically at what happens if we heat or cool inside a parcel. This is a little more detailed version than was given in class.
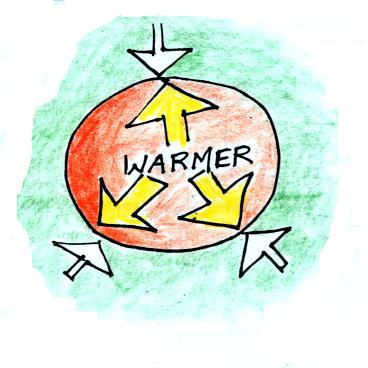
First let's imagine warming the air inside a balloon.

Next we'll warm the air inside the balloon but leave the air outside alone.
 |
 |
Increasing the temperature will momentarily increase the pressure. This creates an imbalance, so this is a temporary situation. Now that P inside is greater than P outside the balloon will expand.

We're left with a balloon that is larger, warmer, and filled with lower density air than it was originally.
 |
 |
The pressures inside and outside are again the same. The pressure inside is again equal to its starting value. The 1st form of the ideal gas law show you that you can increase the temperature and volume of a parcel together in a way that keeps pressure constant (which is what Charles' law requires). The 2nd version of the equation shows that by increasing the temperature and decreasing the density together you can keep pressure constant.
We can go through the same kind of reasoning and see what happens if we cool the air in a parcel.

We'll start with the same picture
we had above.
We'll cool the air inside the parcel. The air outside stays the same.
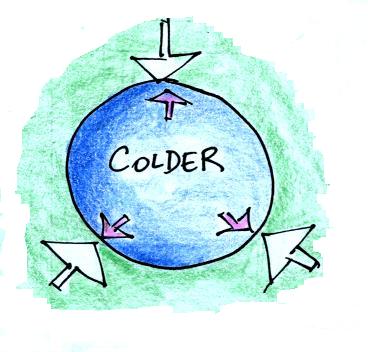
Reducing the air temperature causes the pressure of the air inside the balloon to decrease momentarily. Because the outside air pressure is greater than the pressure inside the balloon the parcel is compressed.

We'll cool the air inside the parcel. The air outside stays the same.
 |
 |
Reducing the air temperature causes the pressure of the air inside the balloon to decrease momentarily. Because the outside air pressure is greater than the pressure inside the balloon the parcel is compressed.

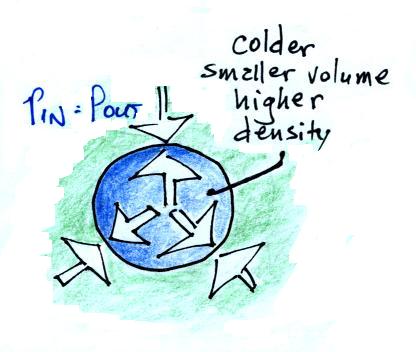
The balloon will get smaller and
smaller (and the pressure inside will get bigger and bigger) until the
pressures inside and outside the balloon are again equal. The
pressure inside is back to the value it had before you cooled the air
in the parcel.
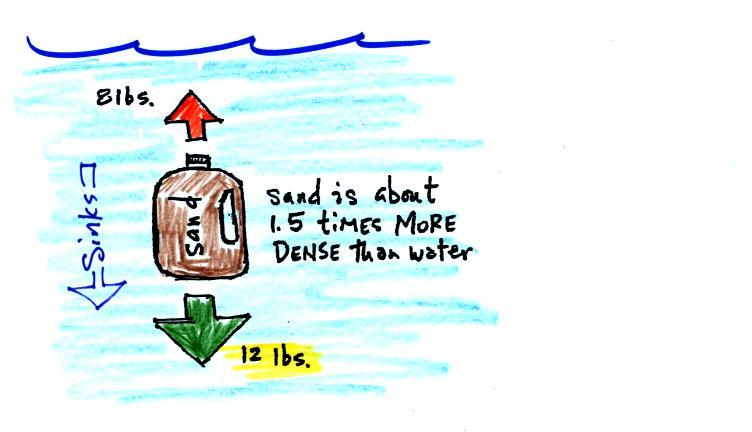
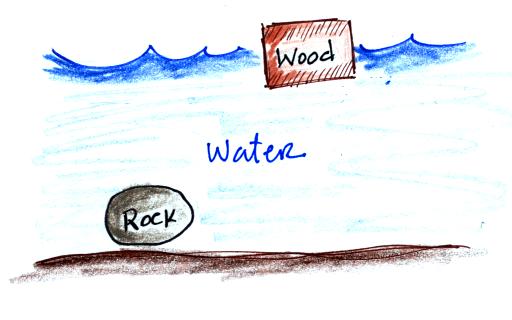
In the atmosphere a parcel of cold air will be high density air.
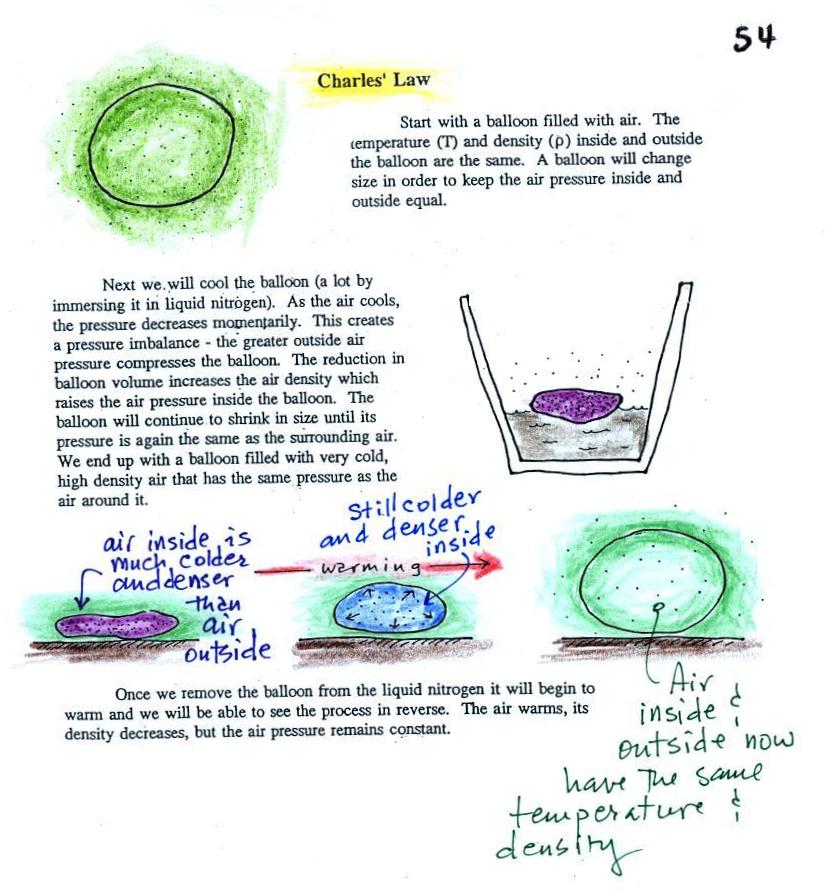
The balloon shrinks down to practically nothing when dunked in the liquid nitrogen. It is filled with very cold, very high density air. When the balloon is pulled from the liquid nitrogen and starts to warm up it expands. Density in the balloon decreases. The volume and temperature keep changing in a way that kept pressure constant (pressure inside the balloon is staying equal to the air pressure outside the balloon). Eventually the balloon ends up back at room temperature (unless it pops while warming up). The air inside the parcel is again equal to the density of the air outside.
And finally the last step toward understanding why warm air rises and cold air sinks. We'll have a look at the forces that act on parcels of air in the atmosphere. This information is found on p. 53 in the photocopied ClassNotes.

Basically it comes down to this - there are two forces acting on a parcel of air in the atmosphere:
First is gravity, it pulls downward. The strength of the gravity force (the weight of the air in the parcel) depends on the mass of the air inside the parcel.
Second there is an upward pointing pressure difference force. This force is caused by the air outside (surrounding) the parcel. Pressure decreases with increasing altitude. The pressure of the air at the bottom of a parcel pushing upward is slightly stronger than the pressure of the air at the top of the balloon that is pushing downward. The overall effect is an upward pointing force.
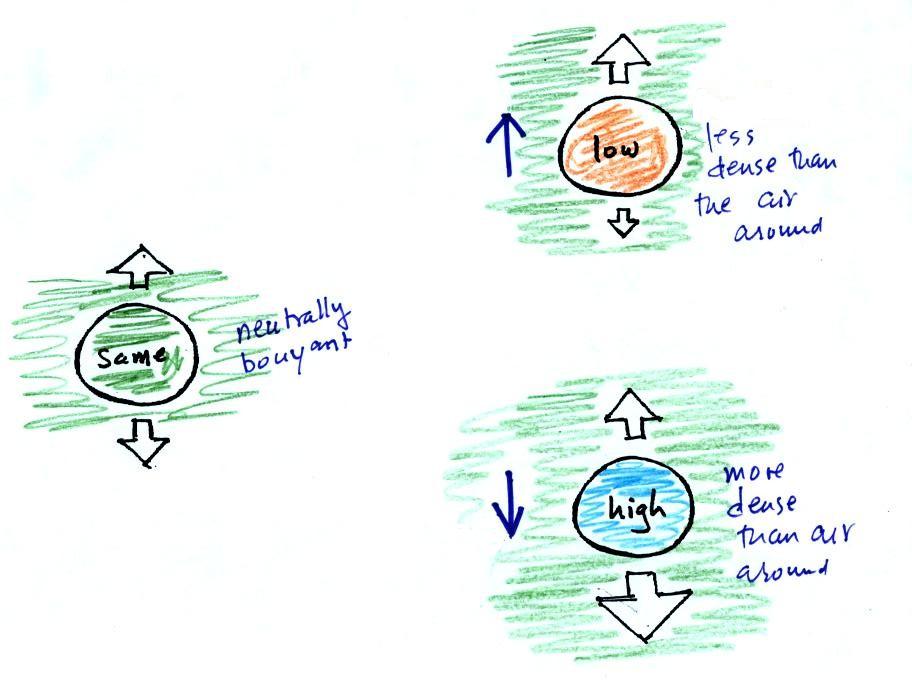
When the air inside a parcel is exactly the same as the air outside, the two forces are equal in strength and cancel out. The parcel is neutrally bouyant and it wouldn't rise or sink, it would just sit in place.
If you replace the air inside the balloon with warm low density air, it won't weigh as much. The gravity force is weaker. The upward pressure difference force doesn't change (because it is determined by the air outside the balloon which hasn't changed) and ends up stronger than the gravity force. The balloon will rise.
Conversely if the air inside is cold high density air, it weighs more. Gravity is stronger than the upward pressure difference force and the balloon sinks.
We did a short demonstration to show how density can determine whether an object or a parcel of air will rise or sink. We used balloons filled with helium (see bottom of p. 54 in the photocopied Class Notes). Helium is less dense than air even when it has the same temperature as the surrounding air. A helium-filled balloon doesn't need to warmed up in order to rise.
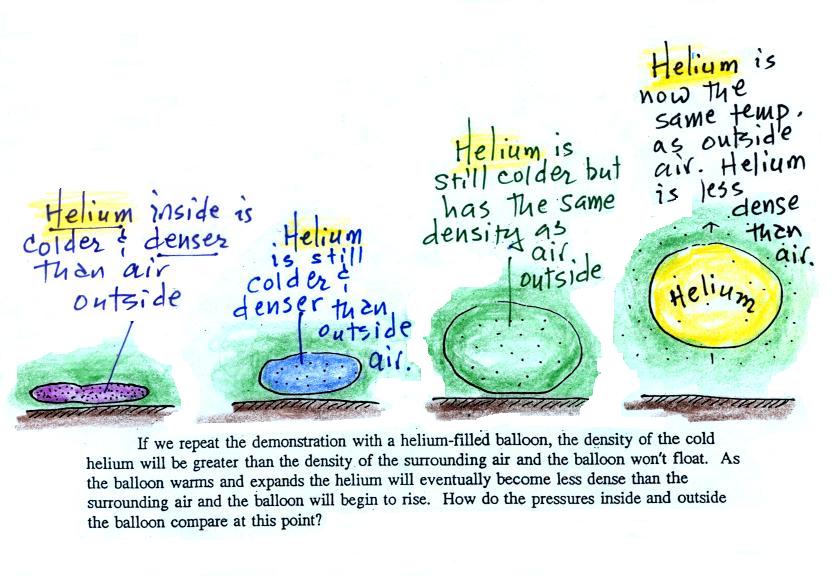
We dunked the helium-filled balloon in some liquid nitrogen to cool it and to cause the density of the helium to increase. When removed from the liquid nitrogen the balloon didn't rise, the gas inside was denser than the surrounding air (the purple and blue balloons in the figure above). As the balloon warms and expands its density decreases. The balloon at some point has the same density as the air around it (green above) and is neutrally bouyant (it's still cooler than the surrounding air). Eventually the balloon becomes less dense that the surrounding air (yellow) and floats up to the ceiling (which in ILC 150 is about 30 feet high)
Something like this happens in the atmosphere (I didn't mention this in class).

Sunlight shines through the atmosphere. Once it reaches the
ground at (1) it is absorbed and warms the
ground. This in turns warms air in contact with the ground
(2) As this air warms, its density starts to decrease. When
the air density is low enough,
small "blobs" of air separate from the air layer at the ground and
begin
to rise, these are called "thermals." (3) Rising air expands and
cools (we've haven't covered
this yet and it might sound a little contradictory). If it cools
enough (to the dew point) a cloud will
become visible as shown at Point 4. This whole process is called
free convection; many of our summer
thunderstorms start this way.
 |
 |
In the atmosphere a parcel of cold air will be high density air.
Charles
Law can be demonstrated by dipping a balloon in
liquid
nitrogen. You'll find an explanation on the top of p. 54 in the
photocopied ClassNotes.

The balloon shrinks down to practically nothing when dunked in the liquid nitrogen. It is filled with very cold, very high density air. When the balloon is pulled from the liquid nitrogen and starts to warm up it expands. Density in the balloon decreases. The volume and temperature keep changing in a way that kept pressure constant (pressure inside the balloon is staying equal to the air pressure outside the balloon). Eventually the balloon ends up back at room temperature (unless it pops while warming up). The air inside the parcel is again equal to the density of the air outside.
And finally the last step toward understanding why warm air rises and cold air sinks. We'll have a look at the forces that act on parcels of air in the atmosphere. This information is found on p. 53 in the photocopied ClassNotes.

Basically it comes down to this - there are two forces acting on a parcel of air in the atmosphere:
First is gravity, it pulls downward. The strength of the gravity force (the weight of the air in the parcel) depends on the mass of the air inside the parcel.
Second there is an upward pointing pressure difference force. This force is caused by the air outside (surrounding) the parcel. Pressure decreases with increasing altitude. The pressure of the air at the bottom of a parcel pushing upward is slightly stronger than the pressure of the air at the top of the balloon that is pushing downward. The overall effect is an upward pointing force.
When the air inside a parcel is exactly the same as the air outside, the two forces are equal in strength and cancel out. The parcel is neutrally bouyant and it wouldn't rise or sink, it would just sit in place.
If you replace the air inside the balloon with warm low density air, it won't weigh as much. The gravity force is weaker. The upward pressure difference force doesn't change (because it is determined by the air outside the balloon which hasn't changed) and ends up stronger than the gravity force. The balloon will rise.
Conversely if the air inside is cold high density air, it weighs more. Gravity is stronger than the upward pressure difference force and the balloon sinks.
We did a short demonstration to show how density can determine whether an object or a parcel of air will rise or sink. We used balloons filled with helium (see bottom of p. 54 in the photocopied Class Notes). Helium is less dense than air even when it has the same temperature as the surrounding air. A helium-filled balloon doesn't need to warmed up in order to rise.

We dunked the helium-filled balloon in some liquid nitrogen to cool it and to cause the density of the helium to increase. When removed from the liquid nitrogen the balloon didn't rise, the gas inside was denser than the surrounding air (the purple and blue balloons in the figure above). As the balloon warms and expands its density decreases. The balloon at some point has the same density as the air around it (green above) and is neutrally bouyant (it's still cooler than the surrounding air). Eventually the balloon becomes less dense that the surrounding air (yellow) and floats up to the ceiling (which in ILC 150 is about 30 feet high)
Something like this happens in the atmosphere (I didn't mention this in class).