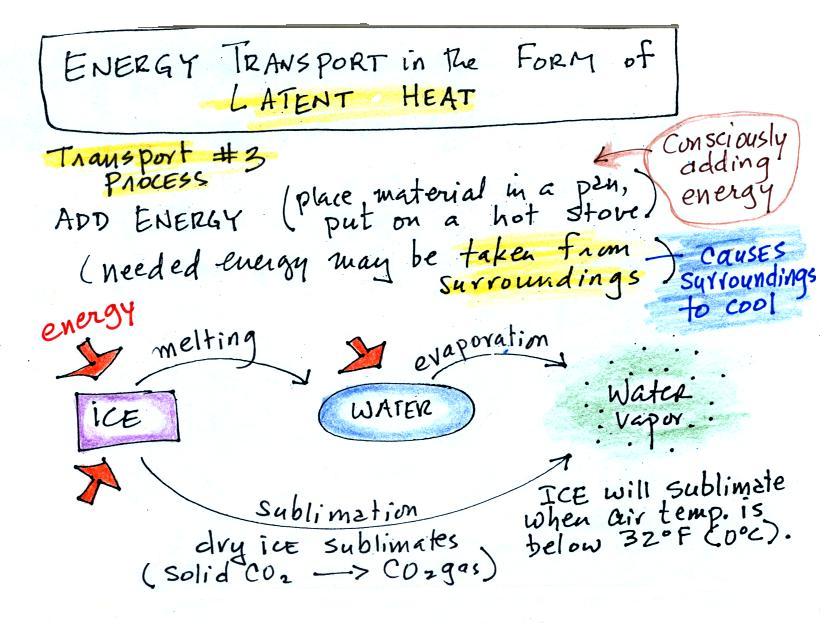
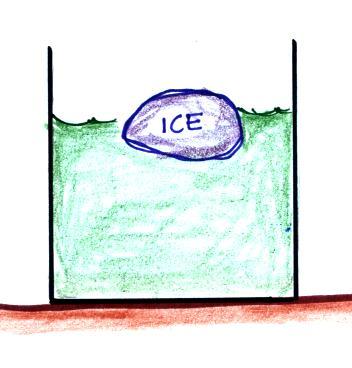
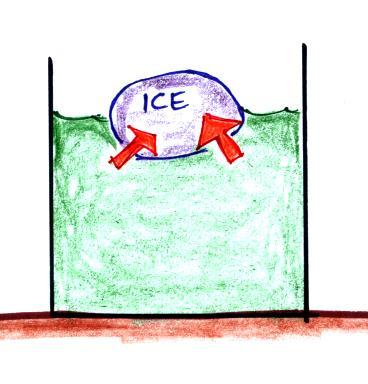
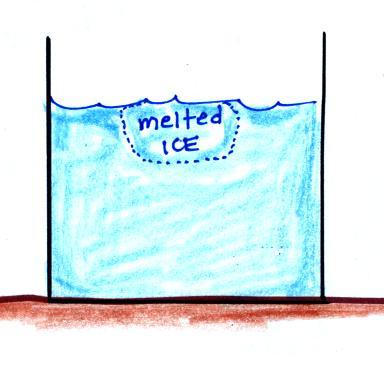
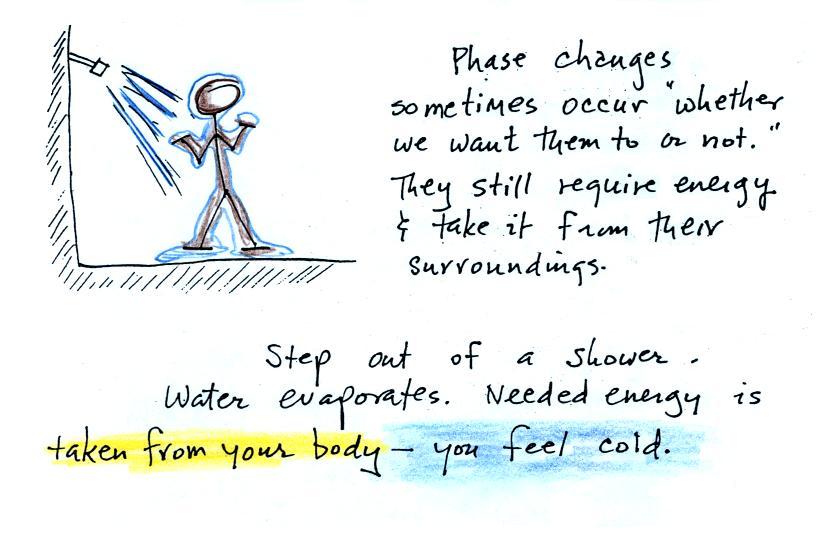
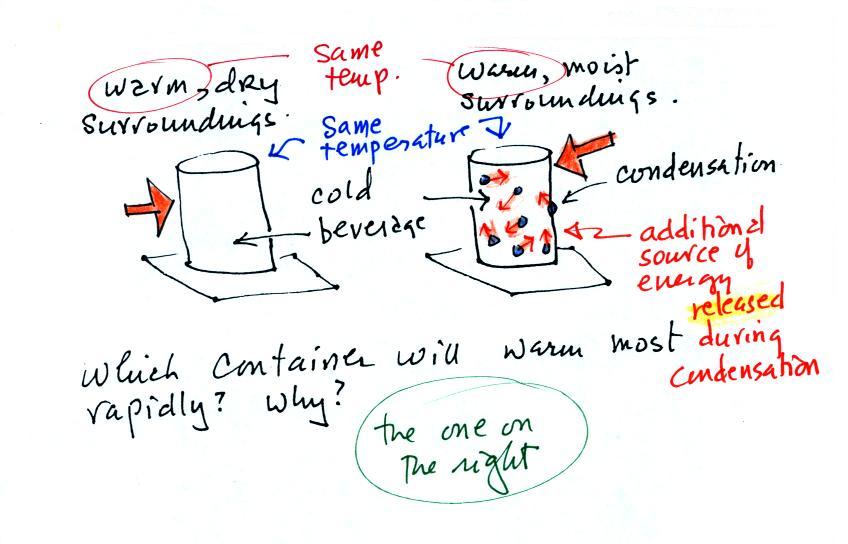
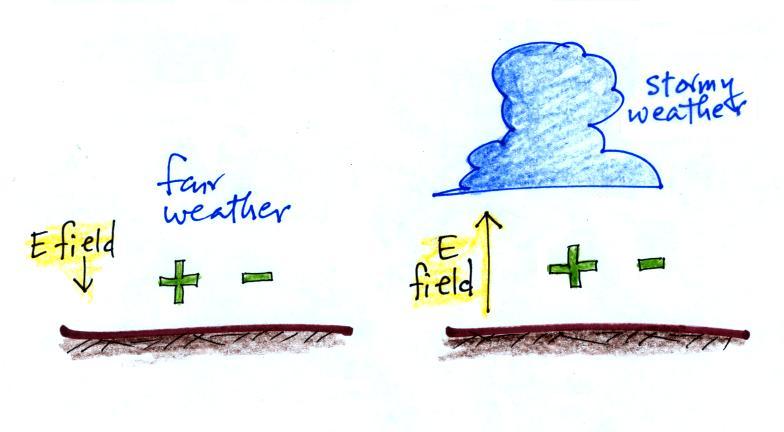
The direction and strength of the E
field near the ground during fair weather and under a thunderstorm are
shown. Show the directions of the forces that would be exerted on
the charges shown in the figure. Click here
when you think you
have the answer.
We'll use this concept of electric field to begin to understand
electromagnetic radiation and how it can transport energy from one
place to another.
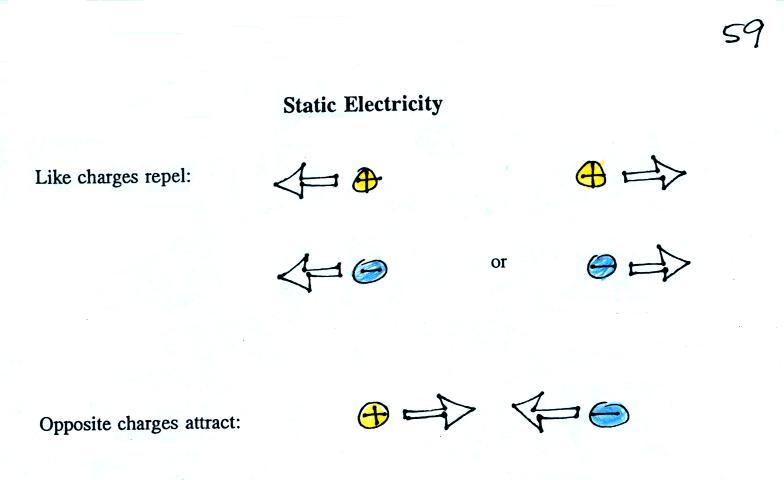
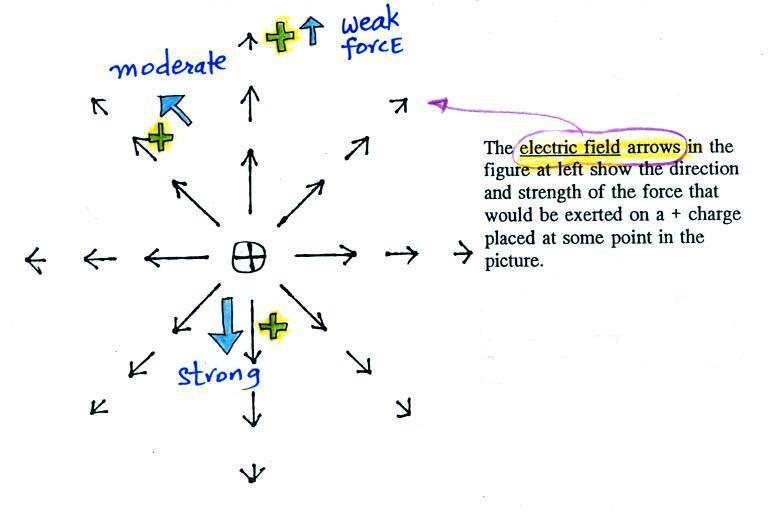
An electric field
arrow
shows the
direction and
gives an idea
of the strength
of the
electrical force
that would be
exerted on a positive charge
You'll find most of the following
on p. 60
in the photocopied ClassNotes. What follows is a little more
detailed explanation than was shown in class.
We imagine turning on a source of
EM radiation and then
a
very short time
later we take a snapshot. In that time the EM radiation has
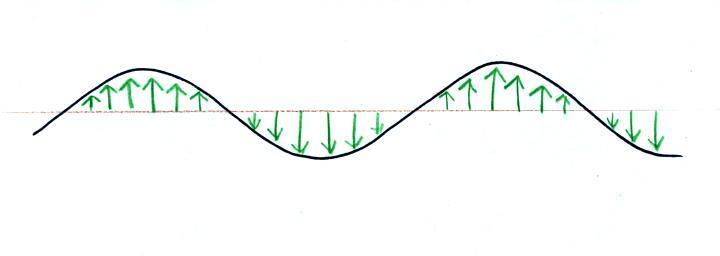
traveled to the right (at the speed of light). The EM radiation
is a wavy pattern of
electric and magnetic field arrows. We'll ignore the
magnetic
field lines. The E field lines sometimes point up, sometimes
down. The pattern of electric field arrows repeats itself.
Textbooks often represent EM
radiation with a wavy line like shown
above. They don't usually explain what the wavy line represents.
The wavy line just connects the
tips of a bunch of electric
field
arrows.
Note the + charge near the right
side of the picture. At the time
this
picture was taken the EM radiation exerts a fairly strong upward force
on
the
+
charge (we use the E field arrow at the location of the + charge to determine the direction
and strength of the force exerted on the + charge).
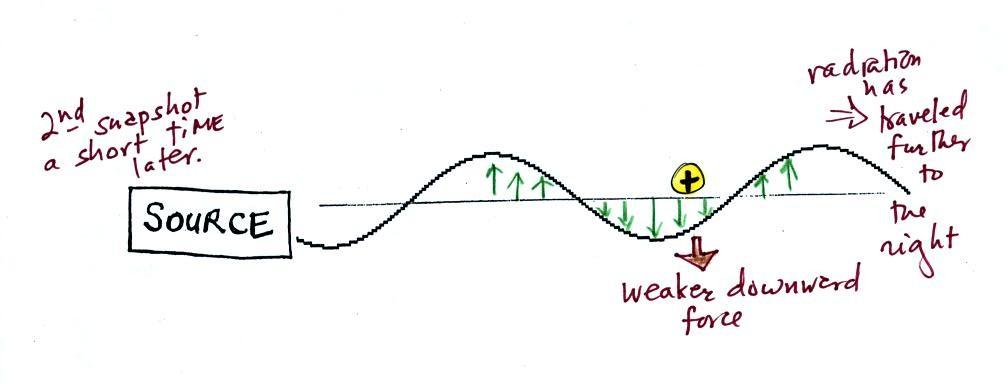
Th picture above was taken a short
time
after the first snapshot aftere the radiation
had
traveled a little further to the right. The EM radiation now
exerts a somewhat weaker downward force on the + charge.
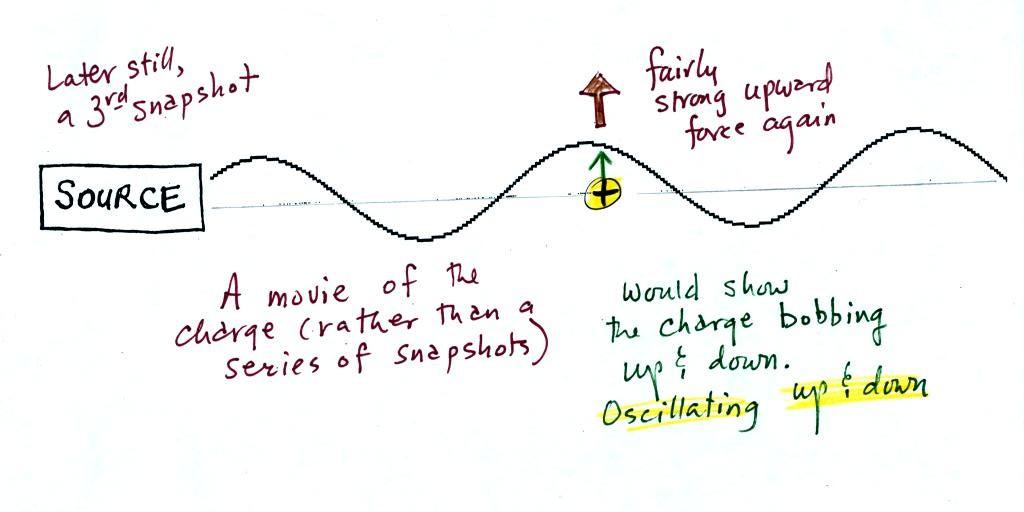
A 3rd snapshot taken a short time
later. The +
charge is now being
pushed upward again.
A
movie
of
the +
charge, rather than just a series of snapshots, would show the
charge
bobbing up and down much like a swimmer in the
ocean would do as waves passed by.
The wavy pattern used to
depict EM radiation can be described spatially (what you would
see in a snapshot) in terms of its
wavelength,
the distance between identical points on the pattern.
Or you can
describe the radiation temporally
using the frequency of oscillation
(number of up and down cycles completed by an oscillating charge per
second). By temporally we mean you look at one particular fixed
point and look at how things change with time.
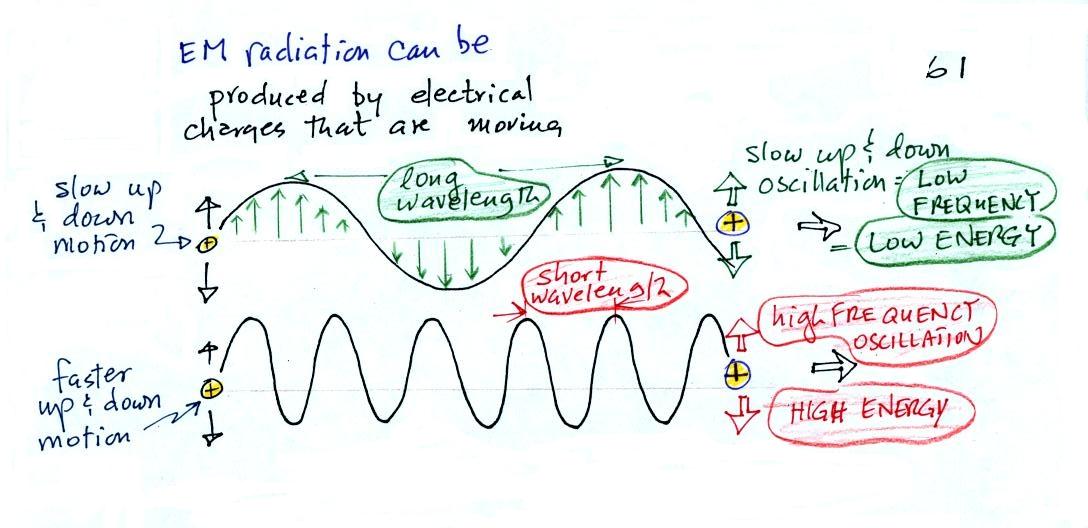
EM radiation can be created when
you cause a charge to move up and
down.
If you move a charge up and down slowly (upper left in the
figure above) you would produce long wavelength radiation that would
propagate out to the right at the speed of light. If you move the
charge up and down more rapidly you produce short wavelength radiation
that propagates at the same speed.
Once the EM radiation encounters the charges at the right side of
the
figure above the EM radiation causes those charges to oscillate up and
down. In the case of the long wavelength radiation the charge at
right oscillates slowly. This is low frequency and low energy
motion. The short wavelength causes the charge at right to
oscillate more rapidly - high frequency and high energy.
These three characteristics: long wavelength / low frequency / low
energy go
together. So do short wavelength / high frequency / high energy.
Note that the two different types of radiation both propagate at the
same speed.
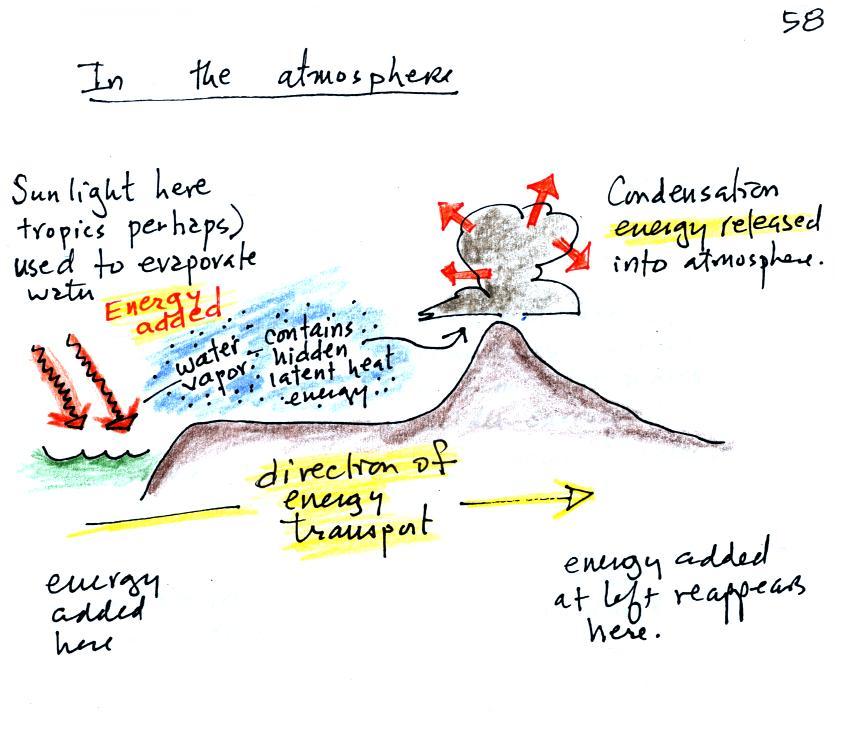
The
following
figure
illustrates how energy can be
transported from one
place to another (even through empty space) in the form of
electromagnetic (EM) radiation.

You add energy when you cause an
electrical charge to move up and down
and create the EM radiation (top left).
In the middle
figure, the EM
radiation that is produced then travels out
to the
right (it could be through empty space or through something like the
atmosphere).
Once
the EM radiation encounters an electrical charge at another location
(bottom right),
the energy reappears as the radiation causes the charge to move.
Energy
has been transported from left to right.
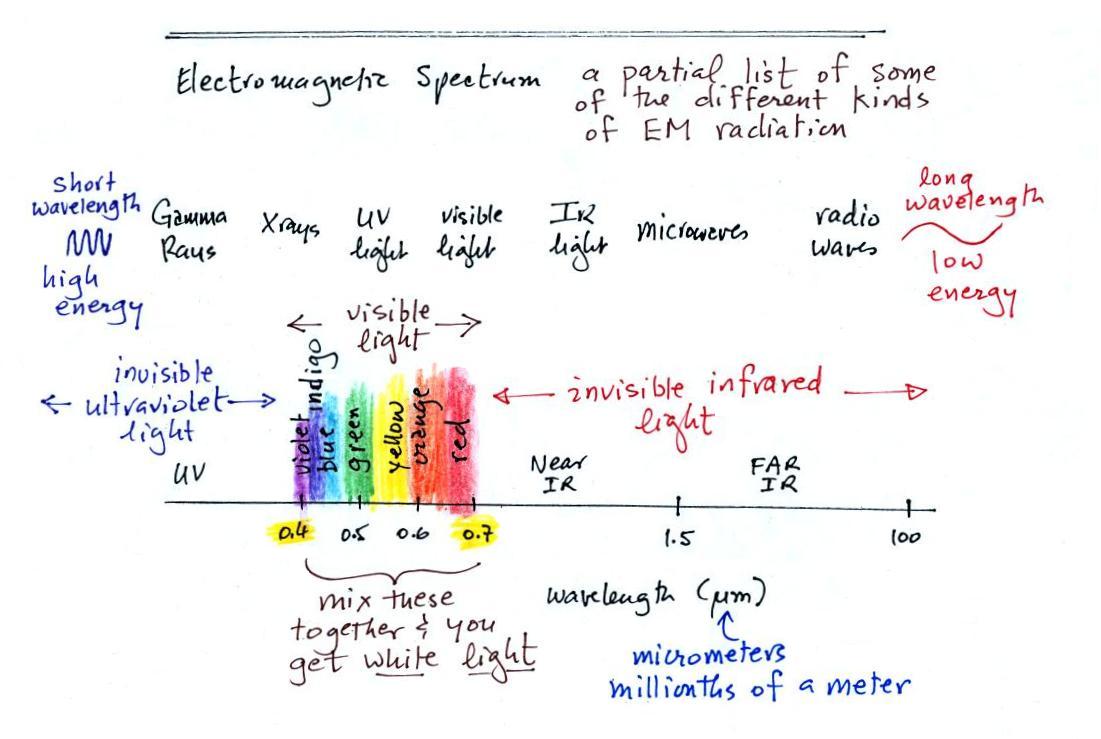
This is really just a partial list
of some of the different
types of EM
radiation. In the top list, shortwave length and high energy
forms of EM radiation are on the left (gamma rays and X-rays for
example). Microwaves and radiowaves are longer wavelength, lower
energy forms of EM radiation.
We will mostly be concerned with just ultraviolet light (UV),
visible
light (VIS), and infrared light (IR). Note the micrometer
(millionths of a meter) units used for wavelength for these kinds of
light. The visible
portion of the spectrum falls between 0.4 and 0.7 micrometers.
UV and
IR light are both invisible. All of the vivid colors
shown above
are just EM radiation with slightly different wavelengths. When
you see all of these colors mixed together, you see white light.
And note that indigio was added to the colors in the spectrum, I
believe it falls between blue and violet (this after a question in the
MWF class). While researching this
I came across the following (compact)
list
of
colors.