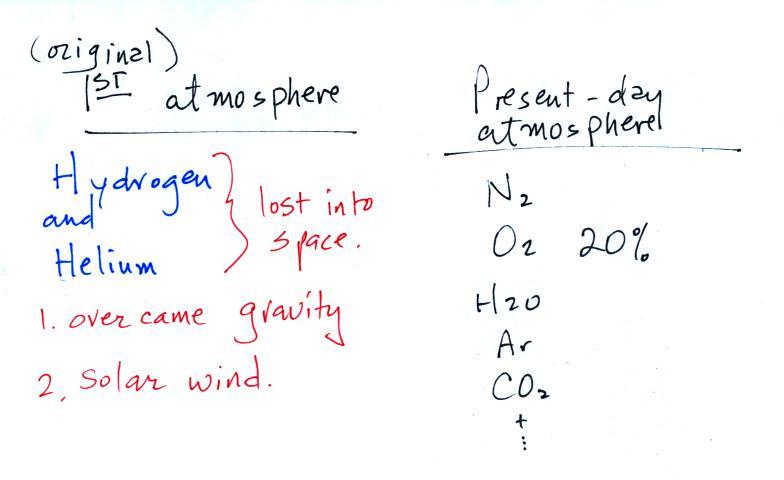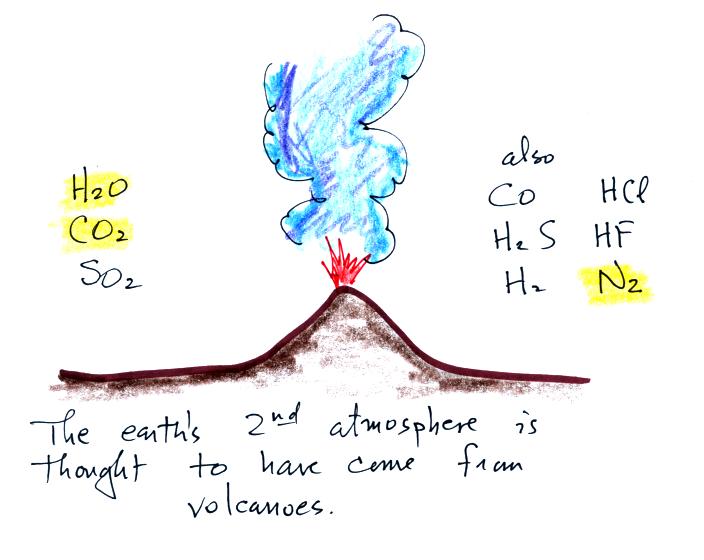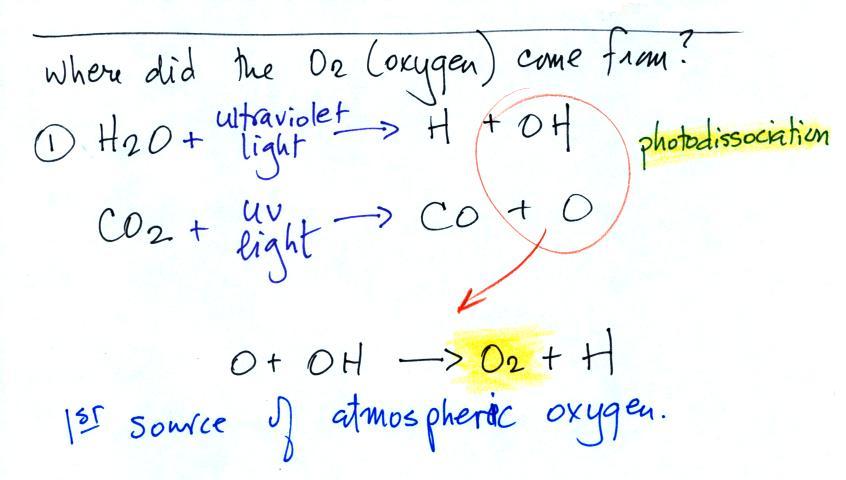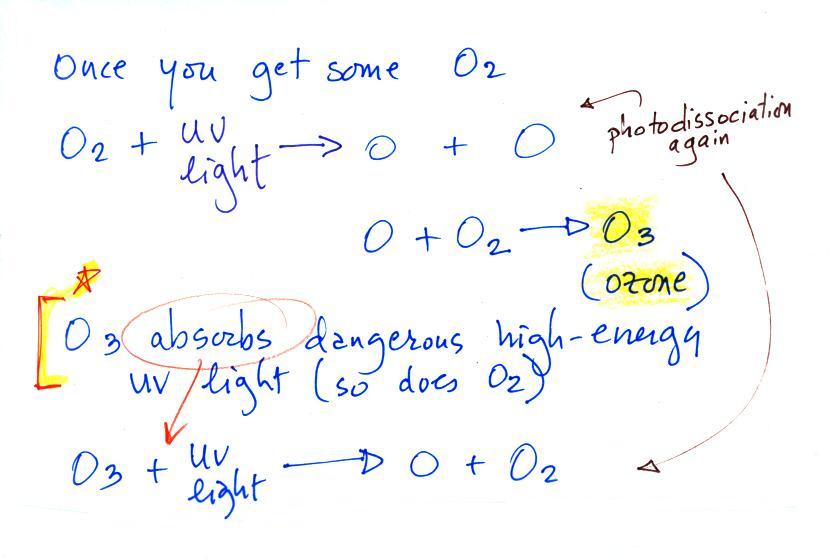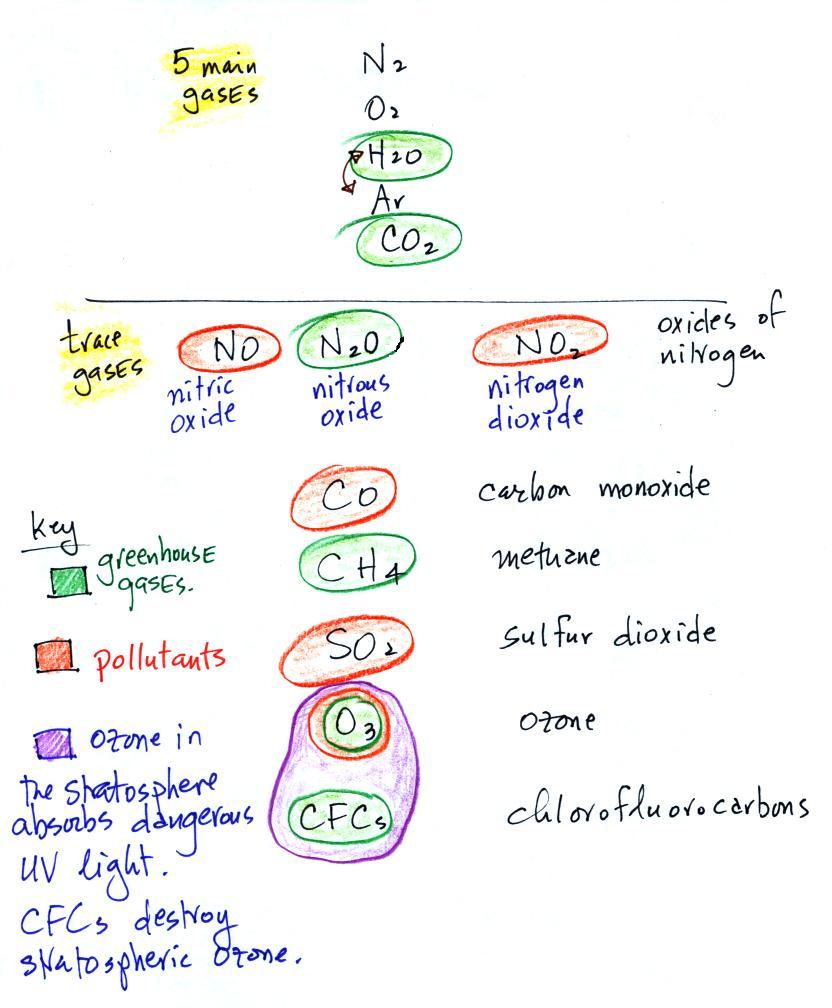
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing
gas),
chlorofluorocarbons,
and
ozone
are
all
greenhouse
gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic and learn more about how the
greenhouse effect actually works later in the course.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants. We'll cover some of
these in more detail today and early next week.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i) Ozone
in the
stratosphere (a layer of the atmosphere between about 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. It was only after ozone started to buildup in the
atmosphere that life could move from the oceans onto land.
Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In
the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
(iii) Ozone is also a greenhouse gas.
This was a
good point for a demonstration, one that was once voted
the prettiest demonstration of the semester.
You are able to see a lot of things in the atmosphere (clouds,
fog, haze, even the blue sky) because of scattering of light.
We'll try to make a cloud of smog in class later this week.
The
individual droplets making up the smog cloud are too small to be seen
by
the
naked eye. But you will be able to see the smog cloud because
the droplets scatter light. So we took some time for a
demonstration that tried to show you
and explain exactly what light scattering is.
In the first part of the demonstration a narrow beam of intense
red
laser light was shined from one side of the classroom to the
other.

The instructor would have been
able
to see the beam if he had stood at the end of the beam of laser light
and looked back along the beam of light toward the laser. That
wouldn't have been a smart thing to do, though, because the beam was
strong
enough to possibly damage his eyes (there's a warning on the
side of the laser).
Everybody was able to see a bright red spot where the laser beam
struck
the wall.

This is because when the intense
beam of
laser light
hits the wall it
is scattered (splattered is a
more descriptive term). The original beam is broken up into a
myriad of weaker rays
of light that are sent out in all directions. There is a ray of
light
sent in the direction of every student in the class. They see the
light because they are looking back in the direction their ray came
from. It is safe to look at this light because the original
intense beam is split up into many much weaker beams.
Next we clapped some erasers together so that some small
particles of chalk dust fell into the laser beam.

Now instead
of a single spot on the wall, students
saws lots of
points of light coming from different positions along a straight
segment of the laser
beam. Each of these points of light was a particle of chalk, and
each piece of chalk dust was intercepting laser light and sending light
out in all directions. Each student saw a ray of light coming
from
each of the chalk particles.
We use chalk because it is white, it will scatter rather
than absorb visible light. What would you have seen if black
particles
of soot had been dropped into the laser beam?
In the last part of the demonstration we made a cloud
by
pouring some water into a cup of liquid nitrogen. The cloud
droplets are much
smaller than the chalk particles but are many more of them. They
make very good scatterers.
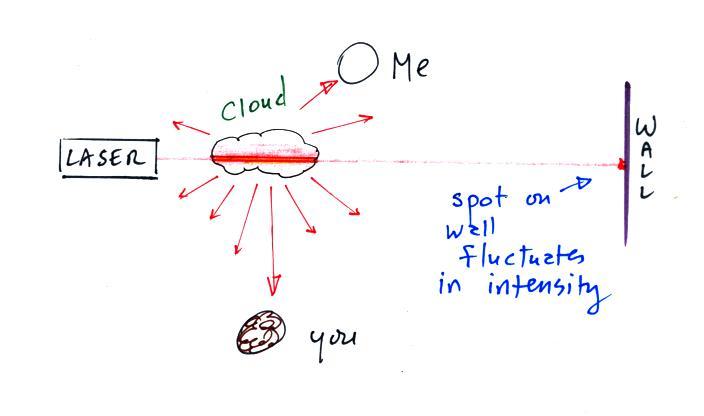
The beam of laser
light really lit up as it passed through the small patches of
cloud. The cloud droplets did a very good job of scattering laser
light. So
much light was scattered
that the spot on the wall fluctuated in intensity (the spot dimmed when
lots of
light was being scattered, and brightened when not as much light was
scattered). Here's a photo I took back in my office.
The laser beam is visible in the
left 2/3 rds of the picture
because it is passing through cloud and light is being scattered toward
the camera. There wasn't any cloud on the right 1/3rd of the
picture so you can't see the laser beam over near Point 1.
There's something else going on in this picture also. We're
not just seeing the narrow beam of laser light but some of the cloud
outside the laser beam is also visible.
Up to this point we've just considered single scattering. A
beam
of light encounters a cloud droplet or a particle of chalk and gets
redirected and then travels all the way to your eye or to a
camera. That's what's happening at Point 2 (it's also shown below
in Path 1). You just see
the narrow laser beam. But sometimes the scattered ray of light
runs into
something else and gets scattered again. This is called multiple
scattering. And that is what is illuminating the cloud alongside
the beam of laser light at Point 3. Light is first scattered by a
cloud droplet in the beam. As it leaves the beam it runs into
another droplet and gets scattered again (Path 2 below). So now
it looks like it
is coming from the cloud surrounding the laser beam rather than from
the beam itself.