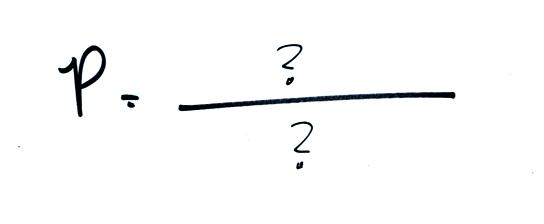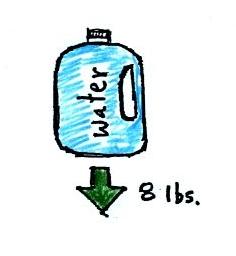Up to this point we
have been thinking of pressure as
being determined
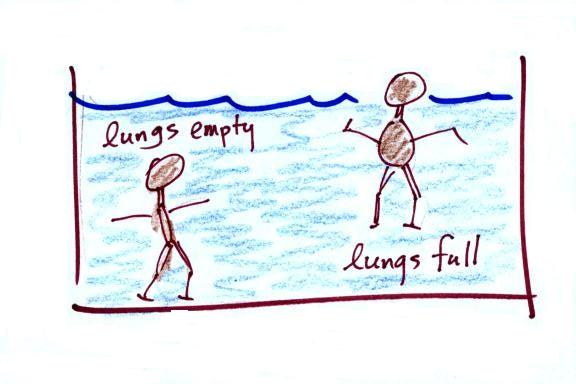
by the weight of the air overhead. Air pressure pushes down
against the ground at sea level with 14.7 pounds of force per square
inch. If you imagine the weight of the atmosphere pushing down on
a balloon sitting on the ground you realize that the air in the balloon
pushes back with the same force. Air everywhere in the atmosphere
pushes upwards, downwards, and sideways.
The ideal gas law
equation is another way of thinking about air pressure, sort of a
microscopic scale view. We ignore
the atmosphere and concentrate on just the air inside a small volume or
balloon or parcel* of air. We are going to "derive" an equation
that shows how pressure (P) depends on certain properties of the air
insidie the balloon.
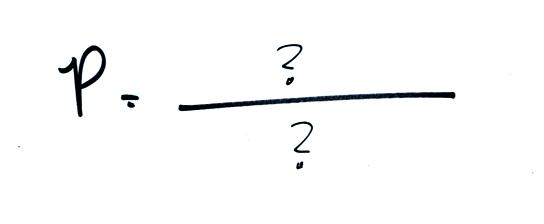
* the word parcel just means a
small volume of air.
Hot air balloons rise (they also
sink), so does the relatively
warm air in a thunderstorm updraft (its warmer than the air around
it). Conversely cold air sinks. The surface winds
caused by a thunderstorm downdraft (as shown above) can reach speeds of
100 MPH and are a serious weather hazard.
Understanding the ideal gas law is
the
first step in explaining what actually causes air to rise or sink.
In the second step we will look
at Charles' Law, a special situation involving the ideal gas law (air
temperature and density change together in a way that keeps the
pressure
inside a balloon constant).
Then we'll learn about the
vertical forces that act on air (an upward
and a downward force) in Step 3.
The figure above makes an important point: the air molecules in a
balloon "filled with air" really take up very little space. A
balloon filled with air is really mostly empty space. It is the
collisions of the air molecules with the inside walls of the balloon
that keep the balloon inflated.
In A
The pressure produced by
the air
molecules inside a balloon will
first depend on the number of air molecules, N, in the balloon.
As you add more and more air to something like a
bicycle tire, the
pressure increases. If there
weren't any air molecules at all there wouldn't be any
pressure. Pressure is directly proportional to N - an
increase in N causes an increase in P. If N doubles, P also
doubles (as long as the other variables in the equation don't change).
In B
Air pressure inside a balloon
also
depends on the size of the
balloon. Pressure is inversely proportional to volume, V
. If V were to double, P would drop to 1/2 its original value.
Note
It
is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
the oxygen concentration experiment described in Week 1. Oxygen
in a
graduated cylinder reacts with steel wool to form rust. Oxygen is
removed from the air sample which is a decrease in N. As oxygen
is removed, water rises up into the cylinder decreasing the air sample
volume. N and V both decrease in the same relative amounts and
the air sample pressure remains constant.
If you were to remove 20% of the air molecules, V would decrease
to 20% of its original value and pressure would stay constant.
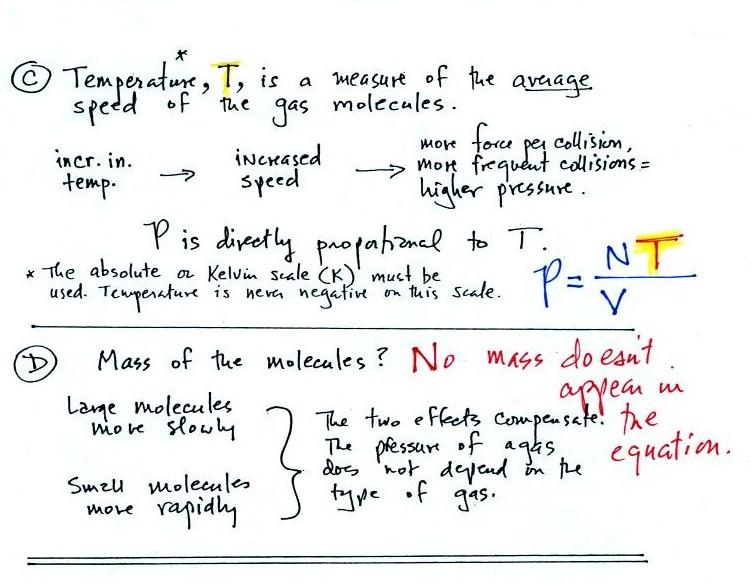
Part C: Increasing
the temperature of the gas in a balloon will cause the gas molecules to
move more quickly. They'll collide with the walls of the balloon
more frequently and rebound with greater force. Both will
increase the pressure. You shouldn't throw a can of spray paint
into a fire because the temperature will cause the pressure inside the
can to increase and the can could explode. We'll demonstrate the
effect of temperature on pressure in class on Friday.
Surprisingly, as explained in Part
D,
the pressure
does
not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
The figure below shows two forms of the ideal gas law. The
top
equation is the one we just derived and the bottom is a second slightly
different version. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers (which we
won't be doing).

Charles' Law is a special case involving the ideal gas law.
Charles Law requires that the pressure in a volume of air remain
constant. T, V, and density can change but they must do so in a
way that keeps P constant. This is what happens in the
atmosphere. Volumes of air in the atmosphere are free to expand
or shrink. They do so to keep the pressure inside the air volume
constant (the
pressure inside the volume is staying equal to the pressure of the air
outside the volume).

Air in the atmosphere behaves like
air in a
balloon. A
balloon can grow or shrink in size depending on the pressure of the air
inside. When a balloon isn't getting bigger or smaller it means
the force inside that is pushing out is balanced by the force outside
that is pushing in.
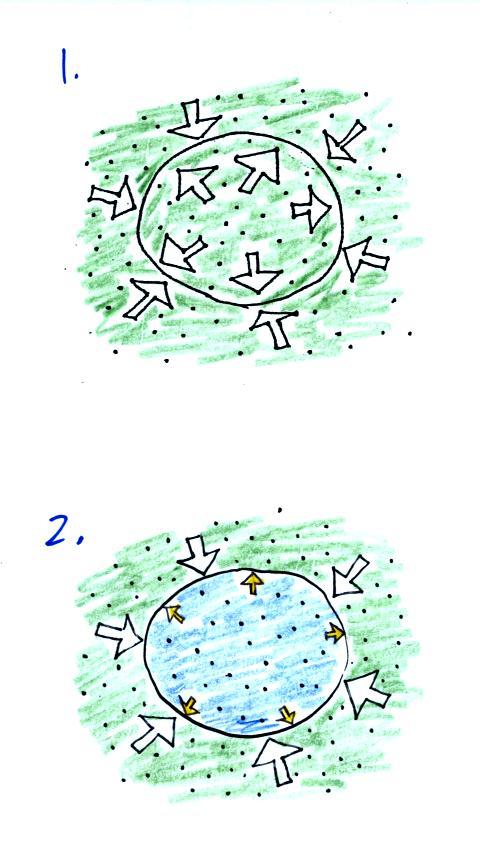
We start in the top figure with air inside a balloon that
is exactly the same as the air outside. The air inside and
outside have been colored green. The arrows show that the
pressure of the air inside pushing outward and the pressure of
the air surrounding the balloon pushing inward are all the same
strength.
Next we warm the air in the balloon (Fig. 2). The ideal gas
law
equation
tells us that the pressure of the air
in the balloon will increase. The increase is
momentary though.
Because the pressure inside is now greater (the big yellow arrows)
than
the pressure outside, the balloon will expand. As volume begins
to increase, the pressure of the air inside the balloon will
decrease.
Eventually the balloon will expand just enough that the pressures
inside and
outside are again in balance. You end up with a balloon of warm
low density air that has the same pressure as the air surrounding it
(Fig. 3)

You can use the same reasoning to
understand what happens when you cool
the air in a balloon.
The air inside and outside are the
same in Fig. 1. Cooling
the
air inside the balloon in Fig. 2 causes a momentary drop in the inside
pressure (small yellow colored arrows) and creates a pressure
imbalance. The stronger outside air
pressure compresses the balloon.
As the balloon volume decreases,
pressure inside the balloon
increases. It eventually is able to balance the outside air
pressure. You end up with a balloon filled with cold high
density air.
If you warm air it will expand and
density will decrease until the
pressure inside and outside the parcel are equal.
If you cool air the parcel will shrink and the density will increase
until the pressures balance.
These two associations:
(i)
warm air = low
density air
(ii) cold air = high density air
are important and will come up a
lot during the remainder of the
semester.
Here's a visual summary of Charles' Law

If you warm a parcel of air the
volume will increase and the density will decrease. Pressure
inside the parcel remains constant. If you cool the parcel of air
it's volume decreases and its density increases. Pressure inside
the parcel remains constant.
Charles
Law is demonstrated in the classroom version of this course by dipping
a balloon in
liquid
nitrogen.

The balloon shrinks down to
practically zero volume when
pulled from the liquid nitrogen. It is filled with very cold high
density air at that point. As
the balloon warms the balloon expands and the density of the air
inside
the balloon decreases. The volume and temperature kept changing
in a way that kept pressure constant. Eventually the balloon ends
up back at room temperature (unless it pops).
Now we are in a position to have a quick look at the forces
that can cause parcels
of air to rise or sink.
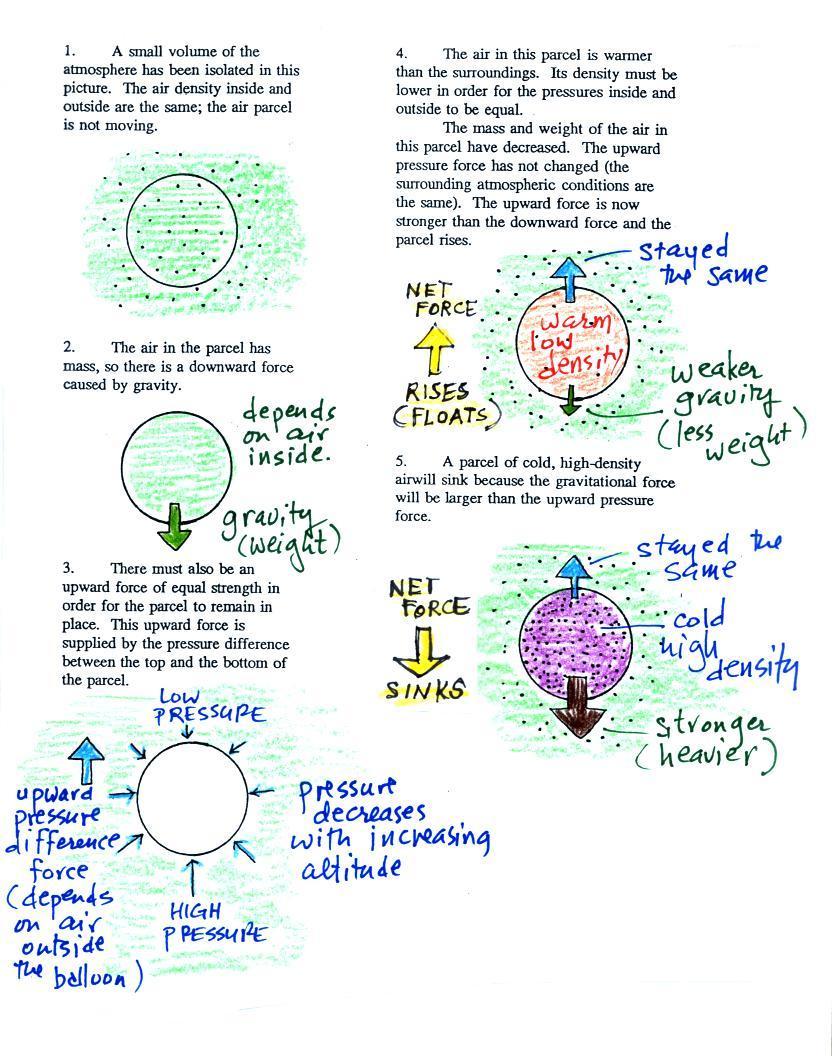
Basically it comes down to this - there are two forces
acting on a parcel of air in the atmosphere:
1. Gravity pulls downward. The strength of the gravity force
depends
on the mass of the air inside
the parcel. This force is just the weight of the parcel
2. There is an upward pointing pressure difference force.
This
force is
caused by the air outside
the parcel (air surrounding the parcel). Pressure decreases with
increasing
altitude. The pressure of the air at the bottom of a parcel
pushing upward is slightly stronger than the pressure of the air at the
top of the balloon that is pushing downward. The overall effect
is an upward pointing force.
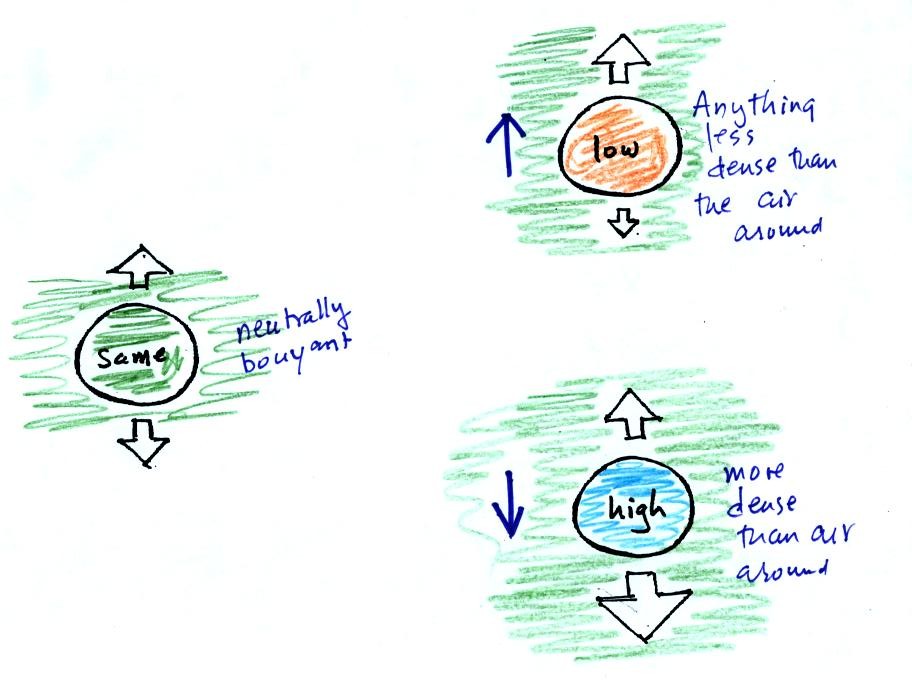
When the air inside a parcel is exactly the same as the air
outside,
the two forces are equal in strength and cancel out. The parcel
is
neutrally bouyant and doesn't rise or sink.
If you replace the air inside the balloon with warm low density
air, it
won't weigh as much. The gravity force is weaker. The
upward
pressure difference force doesn't change, because it is determined by
the air outside the balloon which hasn't changed, and ends up stronger
than the
gravity force. The balloon will rise.
Conversely if the air inside is cold high density air, it weighs
more. Gravity is stronger than the upward pressure difference
force and the balloon sinks.
We
can
modify
the
demonstration that we did earlier to demonstrate
Charles' Law. In this case we use
balloons filled with helium (or hydrogen). Helium is less dense
than air even when the
helium has the same temperature as the surrounding air. A
helium-filled balloon doesn't need to warmed up in order to rise.

We dunk the helium-filled balloon
into some liquid nitrogen to cool
it
and to cause the density of the helium to increase. When
removed
from the liquid nitrogen the balloon doesn't rise, the cold helium gas
is
denser than the surrounding air (the purple and blue balloons in the
figure above). As the balloon warms and expands
its density of the helium decreases. The balloon at some point
has the same
density as the air around it (green above) and is neutrally
bouyant. Eventually the balloon becomes less dense that the
surrounding air (yellow) and floats up to the ceiling.
Something like this happens in the
atmosphere.

The relative strengths of the
downward graviational force and the upward pressure difference force
determine whether a parcel of air will rise or sink. Archimedes
Law is another way of trying to understand this topic.
A gallon of
water weighs about 8 pounds (lbs).
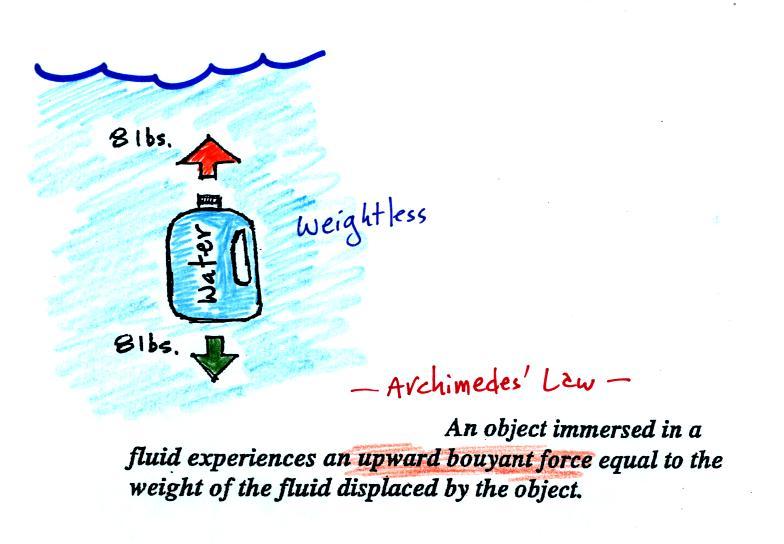
If you submerge a 1 gallon jug of water in a swimming pool, the
jug
becomes, for all intents and purposes, weightless. Archimedes'
Law (see figure below, from p. 53a in the photocopied ClassNotes)
explains why this is true.
The upward bouyant force is really
just another name for the
pressure difference force covered earlier today (higher pressure
pushing
up on the bottle and low pressure at the top pushing down, resulting in
a net upward force). A 1 gallon bottle will displace 1 gallon of
pool water. One
gallon of pool
water weighs 8 pounds. The upward bouyant force will be 8 pounds,
the same as the downward force on the jug due to gravity. The two
forces are equal and opposite.
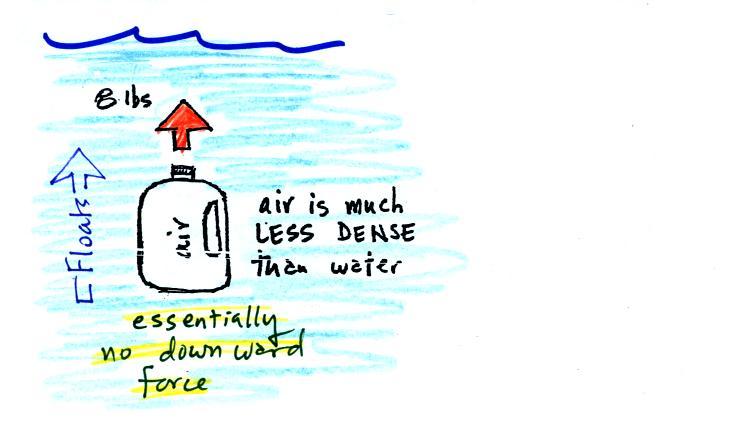
Now we imagine pouring out all the water and filling the 1 gallon
jug
with air. Air is about 1000 times less dense than water;compared
to water, the jug
will weigh practically nothing.
If you submerge the jug in a pool
it will displace 1 gallon of
water
and experience an 8 pound upward bouyant force again. Since there
is no downward force the jug will float.
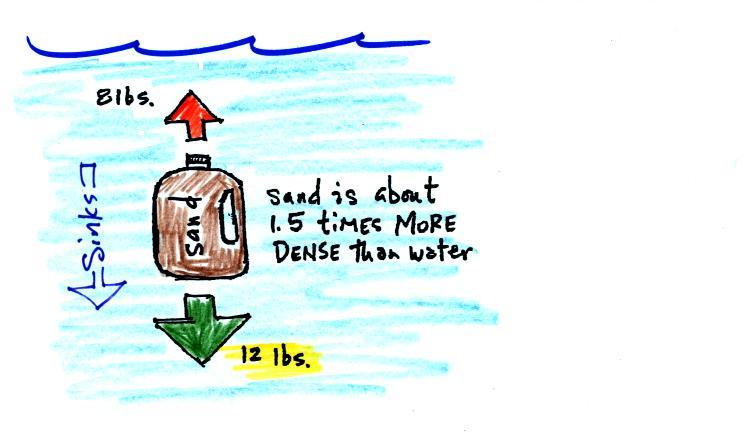
One gallon of sand (which is about 1.5 times denser than water)
jug
will weigh 12 pounds.
The jug of sand will sink because
the downward force is greater
than
the upward force.
You can sum all of this up by saying anything that is less dense
than
water will float in water, anything that is more dense than water will
float in water.
The same reasoning applies to air in the atmosphere.
Air that is less dense (warmer)
than the air around it will
rise.
Air that is more dense (colder) than the air around it will sink.
There's a colorful demonstration of how small
differences in density
can determine whether an object floats or sinks.

Both cans are made of aluminum which has a density almost three times
higher than water. The drink itself is largely water. The
regular Pepsi also has a lot of high-fructose corn syrup, the Diet
Pepsi
doesn't. The mixture of water and corn syrup has a density
greater than plain
water. There is also a little air (or perhaps carbon dioxide gas)
in each can.
The average density of the can of regular Pepsi (water & corn syrup
+
aluminum + air) ends up being slightly greater than the density of
water. The average density of the can of diet Pepsi (water +
aluminum + air) is slightly less than the density of water.
In some respects people in swimming pools are like cans of regular and
diet soda. Some people float (they're a little less dense than
water), other people sink (slightly more dense than water).