Monday Apr. 7, 2014
Mumford and Sons "Little Lion
Man" before class this morning.
The 1S1P Koppen Climate Classification reports were collected
together with Book and Scientific Paper reports. The
Experiment #3 reports turned in last week should be graded in time
to return before the quiz on Wednesday.
Quiz #3 is Wednesday this week. Information about the
reviews can be found at the end of Quiz #3 Study Guide pt.
2. Put some serious study in before coming to the review(s).
We finished the remainder of the material on the ice crystal
process today and looked a some of the different types of
precipitation that can fall from cold clouds.
Here's the outline shown at the beginning of class:
the "heart" of the ice crystal (precipitation
producing) process
snow crystals
ice crystal multiplication
aggregation and snow
riming and graupel
formation of hail
other types of precipitation (rain, virga, sleet, freezing
rain)
infrared satellite photographs
First up, the "tricky" part of
the ice crystal process.
The first figure above (see
p.101 in the photocopied Class Notes) shows a water droplet in
equilibrium with its surroundings. The droplet is
evaporating (the 3 blue arrows in the figure). The rate of
evaporation will depend on the temperature of the water
droplet. There will be some evaporation even from a
droplet that is very cold.
The droplet is surrounded by air that is saturated with water
vapor (the droplet is inside a cloud where the relative humidity
is 100%). This means there is enough water vapor to be
able to supply 3 arrows of condensation. Because the
droplet loses and gains water vapor at equal rates it doesn't
grow or shrink.
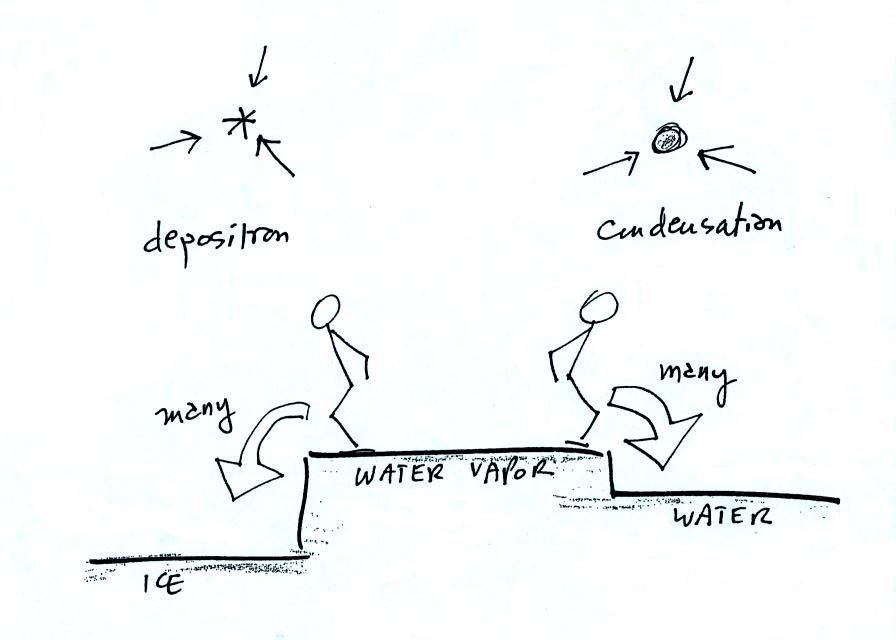
What is needed to keep an ice crystal happy?

We'll assume that everything is
at the same temperature. An ice crystal won't evaporate as
rapidly as a water droplet (only 1 arrow is shown, it could have
been 2 just as long as its not 3). Going from ice to water
vapor is a bigger "jump" than going from water to water
vapor. There won't be as many ice molecules with enough
energy to make that jump. A sort of analogous situation is
shown in the figure below. The class instructor could and
most of the people in the room could jump from the floor to the
top of a 10 or 12 inch tall box. It would be much tougher
to jump to the top of the table (maybe 30 inches off the ground)
or the cabinet (maybe 36 inches) at the front of the room.
There wouldn't be as many people able to do that.
To be in equilibrium the ice crystal only needs 1 arrow of
condensation. There doesn't need to be as much water vapor
in the air surrounding the ice crystal to supply this lower rate
of condensation.
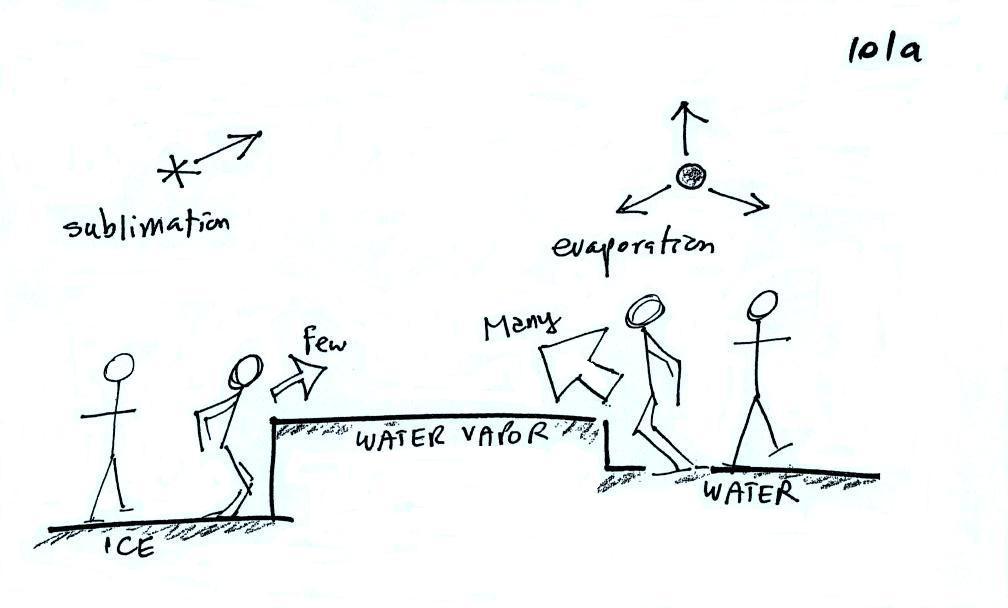
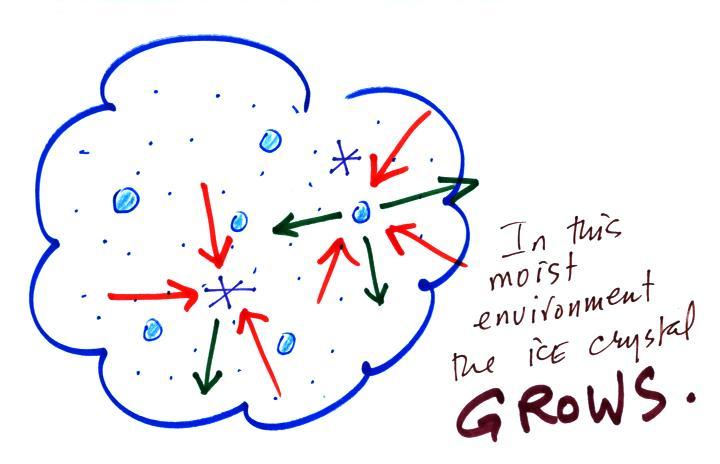
Now what happens in the mixed phase region of a cold
cloud is that ice crystals find themselves in the very moist
surroundings needed for water droplet equilibrium. This is shown
below.
The water droplet is in equilibrium (3 arrows of evaporation and
3 arrows of condensation) with the surroundings. The ice
crystal is evaporating more slowly than the water droplet.
Because the ice crystal is in the same surroundings as the water
droplet water vapor will be condensing onto the ice crystal at
the same rate as onto the water droplet. The ice crystal isn't in
equilibrium, condensation (3 arrows) exceeds evaporation (1
arrow) and the ice crystal will grow. That's what
makes the ice crystal process work.
The equal rates of condensation are shown in the figure
below using the earlier analogy.
Most everyone can manage to make the big or the small jump down.
Now
we will see what can happen once the ice crystal has had a
chance to grow a little bit.
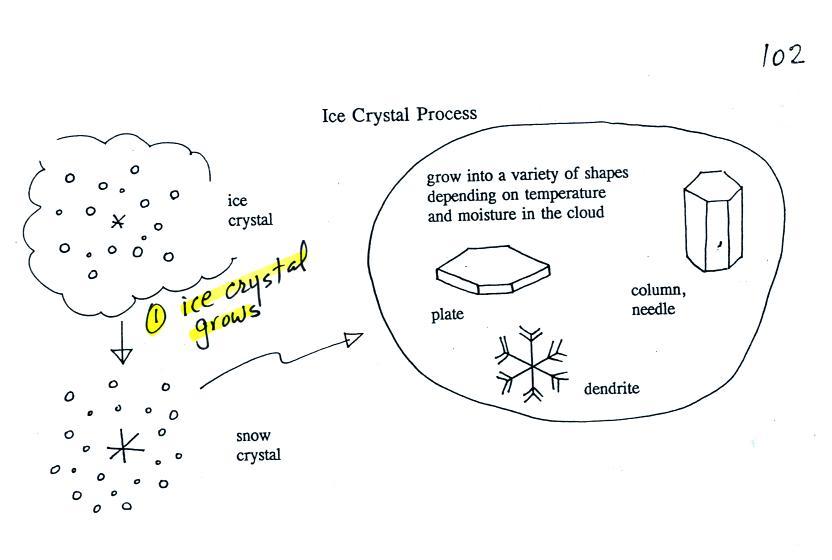
Once an ice crystal has grown it becomes
a snow crystal (this figure is on p. 102 in the
ClassNotes). Snow crystals can have a variety of shapes
(plates, dendrites, columns, needles, etc.; these are called
crystal habits) depending on the conditions (temperature and
moisture) in the cloud. Dendrites are the most common
because they form where there is the most moisture available
for growth. With more raw material available it makes
sense there would be more of this particular snow crystal
shape.
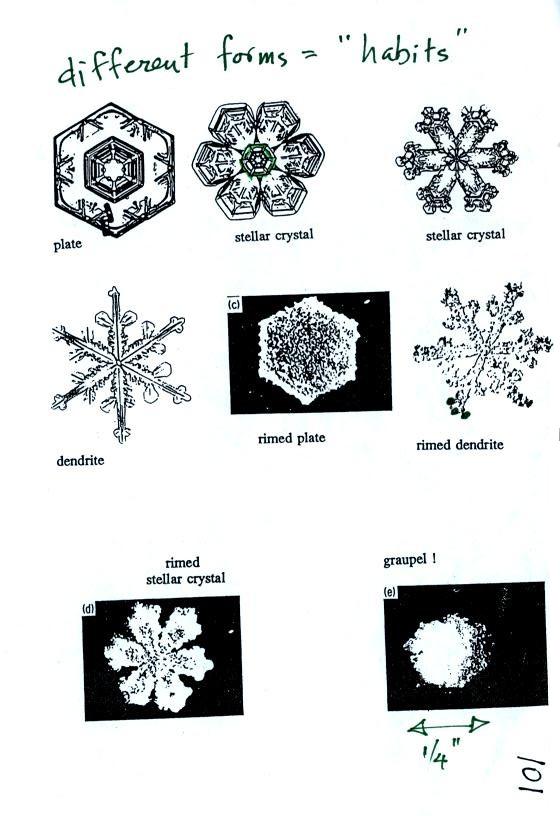
Here
are some actual photographs of snow crystals (taken with a
microscope). Snow crystals are usually 100 or a few 100s
of micrometers in diameter (tenths of a millimeter in
diameter). The different shapes are called "habits".
You'll find some much better photographs and a pile of
additional information about snow crystals at www.snowcrystals.com.
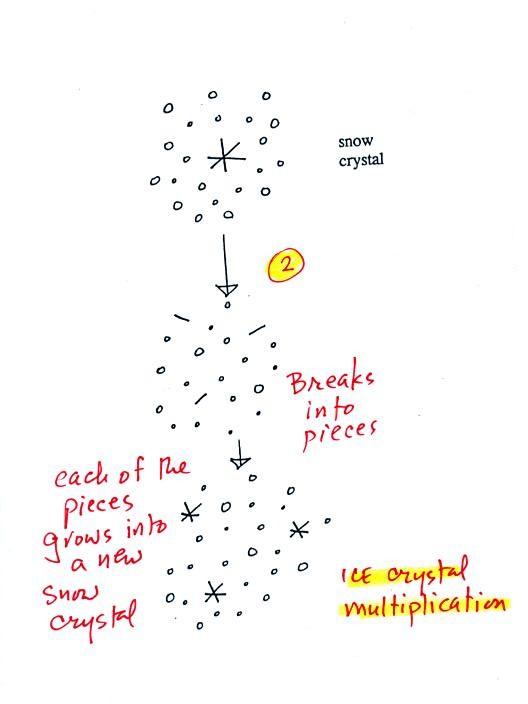
A variety of things can happen once a
snow crystal forms. First it can break into pieces, then
each of the pieces can grow into a new snow crystal.
Because snow crystals are otherwise in rather short supply,
ice crystal multiplication is a way of increasing the amount
of precipitation that ultimately falls from the cloud.

Several snow crystals can collide and
stick together to form a snowflake. Snow crystals are
small, a few tenths of a millimeter across. Snowflakes
can be much larger and are made up of many snow crystals stuck
together. The sticking together or clumping together of
snow crystals is called aggregation (I frequently forget the
name of this process and don't expect you to remember it
either).
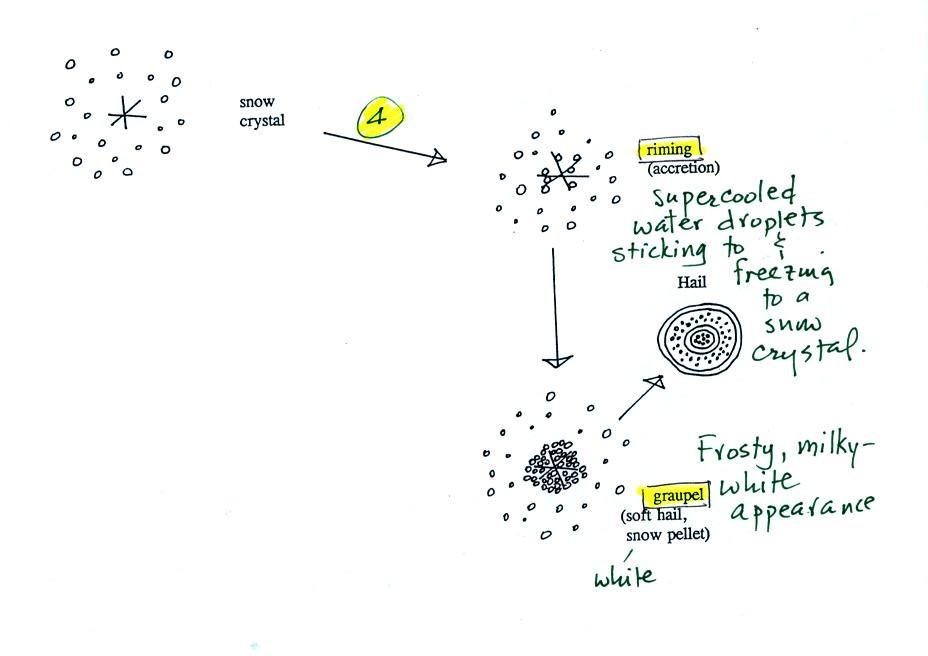
The next process and particle are
something that I hope you will remember.
Snow crystals can collide with
supercooled water droplets. The water droplets may stick
and freeze to the snow crystal. This process is called
riming or accretion (note this isn't called collision
coalescence even though it is the same idea). If a snow
crystal collides with enough water droplets it can be
completely covered with ice. The resulting particle is
called graupel. Graupel is sometimes mistaken for hail
and is called soft hail or snow pellets. Rime ice has a
frosty milky white appearance. A graupel particle
resembles a miniature snow ball. Graupel particles
often serve as the nucleus for a hailstone.
Graupel is made of milky white frosty
rime ice. Sleet, we will find, is made of clear
ice. Here are some pictures to help you better
appreciate the differences in appearance.

Here's a snowball. It's white
and you can't see through it. It's made up of lots
of smaller crystals of ice. Graupel is just a
small snowball.
source
|

The ice in a snow cone is basically
the same. Lots of smaller chunks of ice.
The ice is frosty white (before you added the flavored
syrup. )
source
|
Graupel is sometimes referred as
snow pellets. Sleet is sometimes called ice pellets.
clear transparent crystals of sugar
source of
this photograph
|

sugar cubes are made up of many much smaller grains of
sugar and have a frostly white appearance.
|
Graupel is often mistaken for
hail. This figure gives you an idea of how hail forms.

In
the figure above a hailstone starts with a graupel particle
(Pt. 1, colored green to represent rime ice). The
graupel falls or gets carried into a part of the cloud where
it collides with a large number of supercooled water droplets
which stick to the graupel but don't immediately freeze.
The graupel gets coated with a layer of water (blue) at Pt.
2. The particle then moves into a colder part of the
cloud and the water layer freeze producing a layer of clear
ice (the clear ice, colored violet, has a distinctly different
appearance from the milky white rime ice), Pt. 3. In Tucson this is often the only example of hail
that you will see: a graupel particle core with a single layer
of clear ice.
In the severe thunderstorms in the Central Plains, the
hailstone can pick up additional layers of rime ice and clear
ice and hailstones can be composed of many alternating layers of
rime and clear ice. An unusually large hailstone
(around 3 inches in diameter) has been cut in half to show
(below) the different layers of ice. The picture below
is close to actual size. If something like this were to
hit you in the head it would split your skull open.
Here's some pretty good video of a hailstorm
in Phoenix.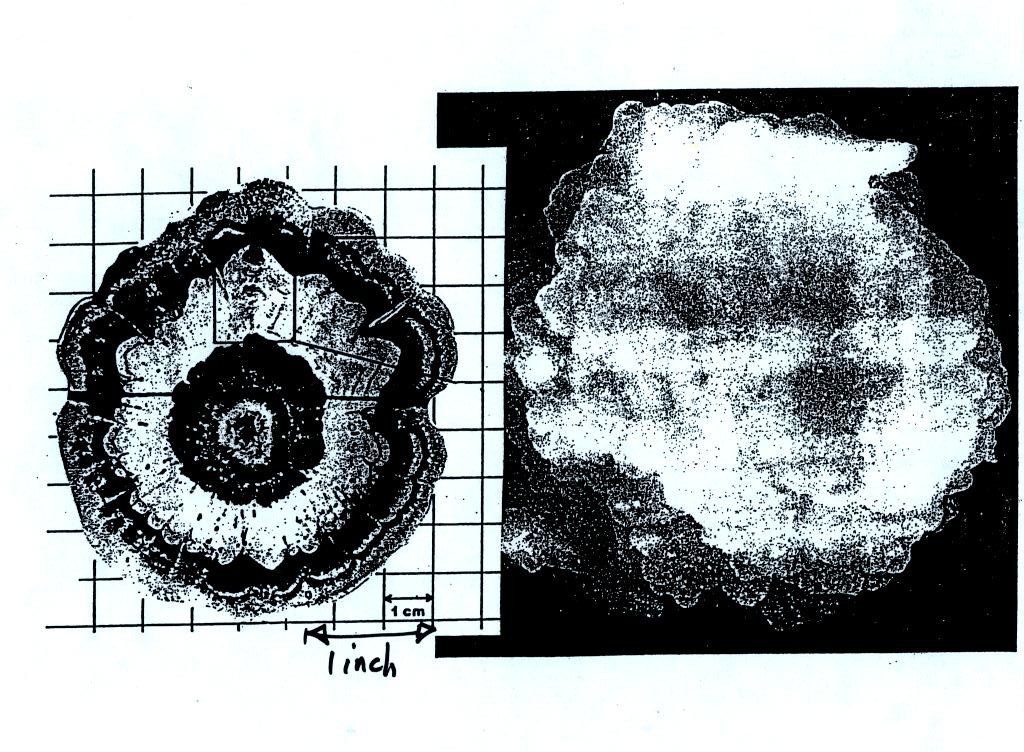
Hail is produced in strong thunderstorms
with tilted updrafts. You would never see hail (or
graupel) falling from a nimbostratus cloud. A new record
was apparently set for a
large hailstone in Hawaii in March of this year.
Hawaii is an unusual place for hail this large to be found.
Hail is produced by thunderstorms (cumulonimbus clouds) because
the cloud is thick and thunderstorms have strong updrafts that can
keep the hailstone in the cloud where it can grow. Hail
would never fall from a nimbostratus cloud they are too thin and
the updrafts are too weak. The
figure below wasn't shown in class.
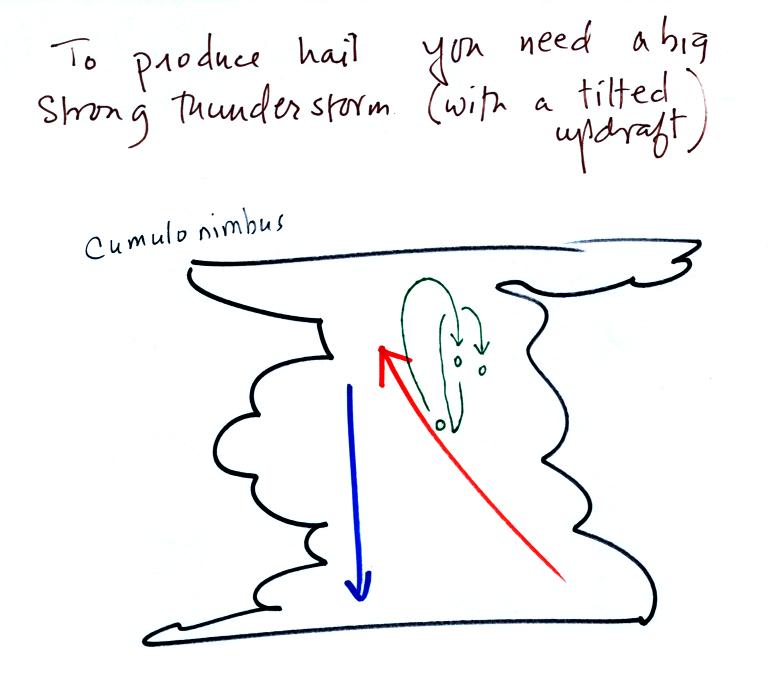
This thunderstorm has a tilted
updraft. The growing hailstone can fall back into the
updraft (rather than falling out of the cloud) and be carried
back up toward the top of the cloud. In this way the
hailstone can complete several cycles through the interior of
the cloud.
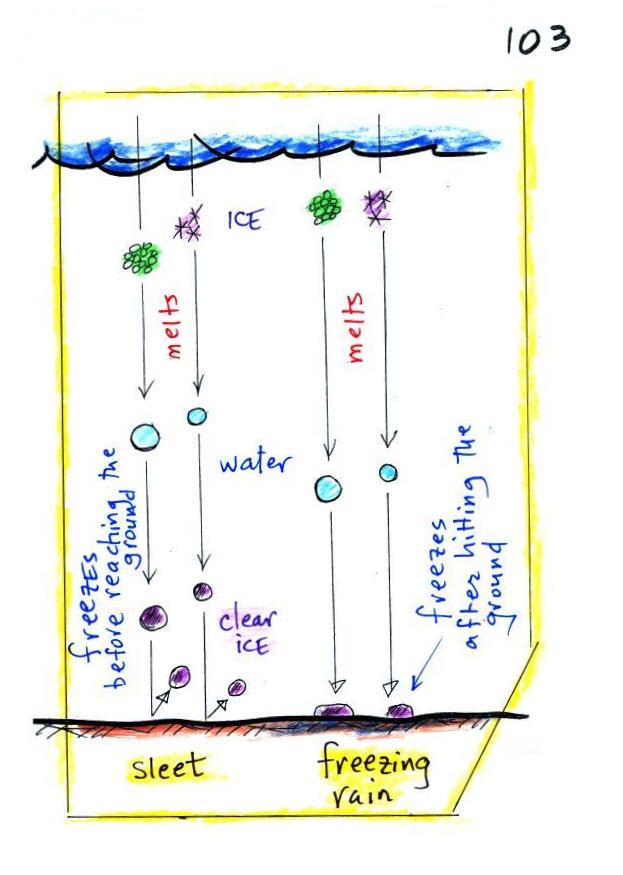
Finally on p. 103 in the
ClassNotes are illustrations of some of the things that can
happen once a precipitation particle falls from a cloud.
I've split this into two groups for clarity.

Essentially all the rain that
falls in Tucson is produced by the ice crystal process.
The left figure above shows how this happens. A falling
graupel particle or a snow flake moves into warmer air and
melts. The resulting drops of water fall the rest of the
way to the ground and would be called RAIN.
In the middle picture graupel particles can survive the trip
to the ground without melting even in the summer. Many
people on the ground would call this hail but that wouldn't be
quite right. Graupel is less common in the winter because
it comes from thunderstorms and they don't form very often in
the winter. Snow can survive the trip to the ground in the
winter but not the summer.
Sometimes the falling raindrops will evaporate before reaching
the ground. This is called VIRGA and is pretty common
early in the summer thunderstorm season in Arizona when the air
is still pretty dry. Lightning that comes from
thunderstorms that aren't producing much precipitation is called
"dry lightning" and often starts brush fires.
Last, before we leave the topic of clouds and precipitation,
some information about satellite photographs of clouds.
We only had time for the material on IR satellite photographs
but I've added information on visible photographs also (visible
satellite photographs of clouds won't be covered on the
quiz). The figure below wasn't
shown in class.
IR satellite photographs
When you see satellite photographs of clouds on the TV weather
you are probably seeing an infrared satellite photograph.
1.
An infrared satellite photograph detects the 10 μm
IR radiation actually emitted by the ground, the ocean and by
clouds. You don't depend on seeing reflected sunlight, so clouds can be
photographed during the day and at night, 24 hours per
day. You may recall that 10 μm radiation is in the middle of
the atmospheric window, so this radiation is able to pass through
air without being absorbed. If clouds don't get in the way,
you can see the ground and the ocean on an IR photograph.
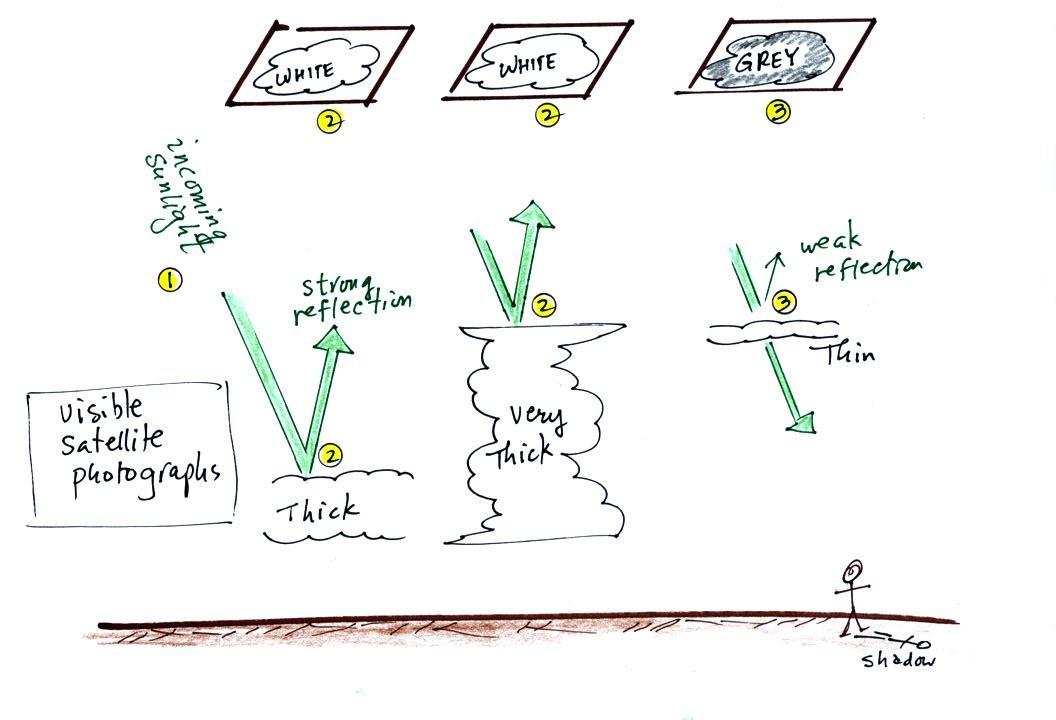
2. Clouds do absorb 10 μm
radiation and then reemit 10 μm IR
radiation upwards toward the satellite and down toward the
ground. It is the radiation emitted by the top surface of
the cloud that will travel through the atmosphere and up to the
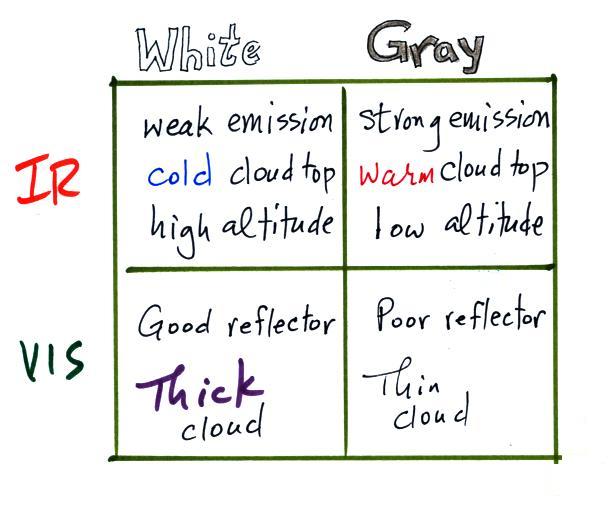
satellite. The top surface of a low altitude cloud will be
relatively warm. Warmer objects emit stronger IR radiation
than a cool object (the Stefan Boltzmann law). This is shown
as grey on an IR satellite photograph. A grey
unimpressive looking cloud on an IR satellite photograph may
actually be a thick nimbostratus cloud that is producing rain or
snow.
3. Cloud tops found at high altitude are cold and
emit IR radiation at a lower rate or lower intensity. This
shows up white on an IR photograph.
4. Two very different clouds (a thunderstorm and a
cirrostratus cloud) would both appear white on the satellite
photograph and would be difficult to distinguish.
Meteorologists are interested in locating thunderstorms because
they can produce hazardous severe weather. This can't be
done using just IR photographs.
5. And here is what I think is one of the most
interesting things you can see on an IR satellite photograph, it
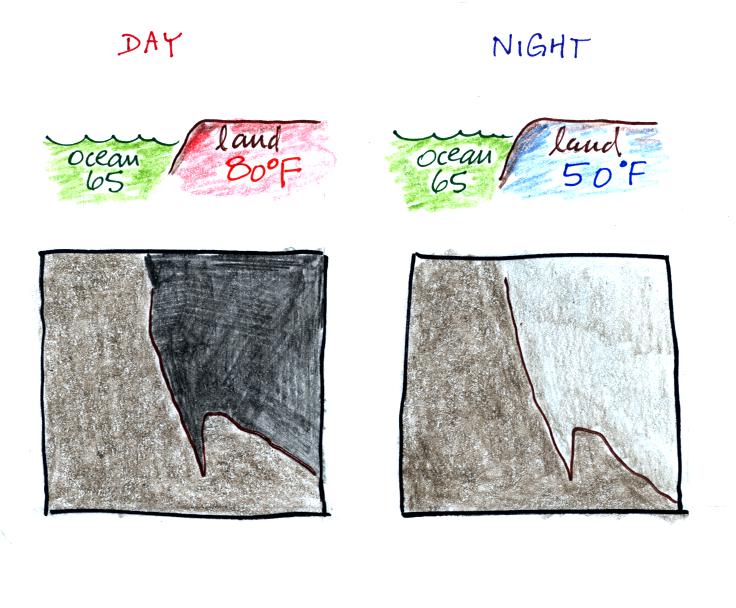
doesn't have anything to do with clouds. The ground changes
temperature during the course of the day. On an infrared
satellite animation you can watch the ground change from dark grey
or black (afternoon when the ground is warmest) to lighter grey
(early morning when the ground is cold) during the course of a
day. Because of water's high specific heat, the ocean right
alongside doesn't change temperature much during the day and
remains the same shade of grey throughout the day.
Here's a
link to an IR satellite photograph loop on the UA
Atmospheric Sciences Dept. webpage. Watch the ground
change from light grey to dark grey. By comparison the
ocean's shade of grey doesn't appear to change at all.
24 hours of IR
satellite photography (National Weather Service)
The remaining material
wasn't covered in class
Visible satellite photographs
1. A visible satellite photograph photographs sunlight that
is reflected by clouds. It shows what you would see if you
were out in space looking down at the earth. You won't see
clouds on a visible satellite photograph at night.
2. Thick clouds are good reflectors and appear white. The
low altitude layer cloud and the thunderstorm would both appear
white on this photograph and would be difficult to distinguish.
3. Thinner clouds don't reflect as much light and appear
grey.
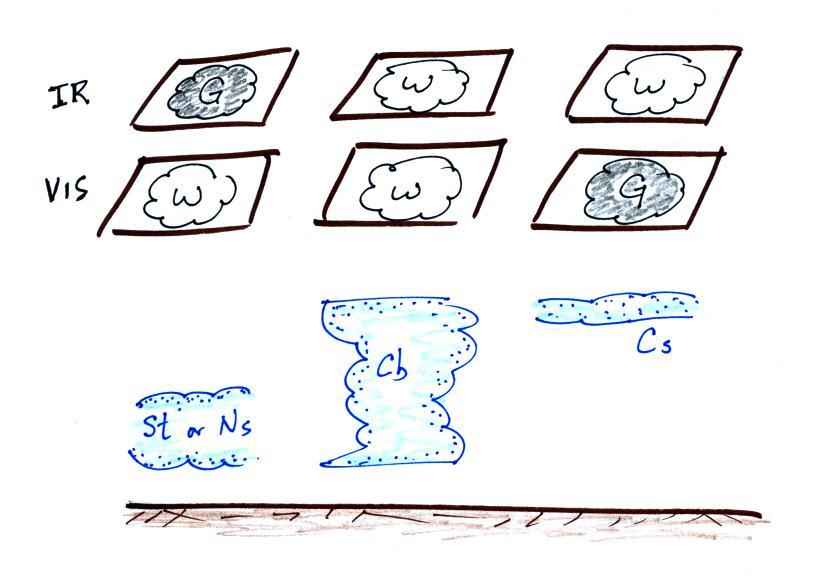
Here's a summary
The figure below shows how if you
combine both visible and IR photographs you can begin to
distinguish between different types of clouds.
You can use this figure to answer the satellite
photograph question that is on the Quiz #3 Study Guide.