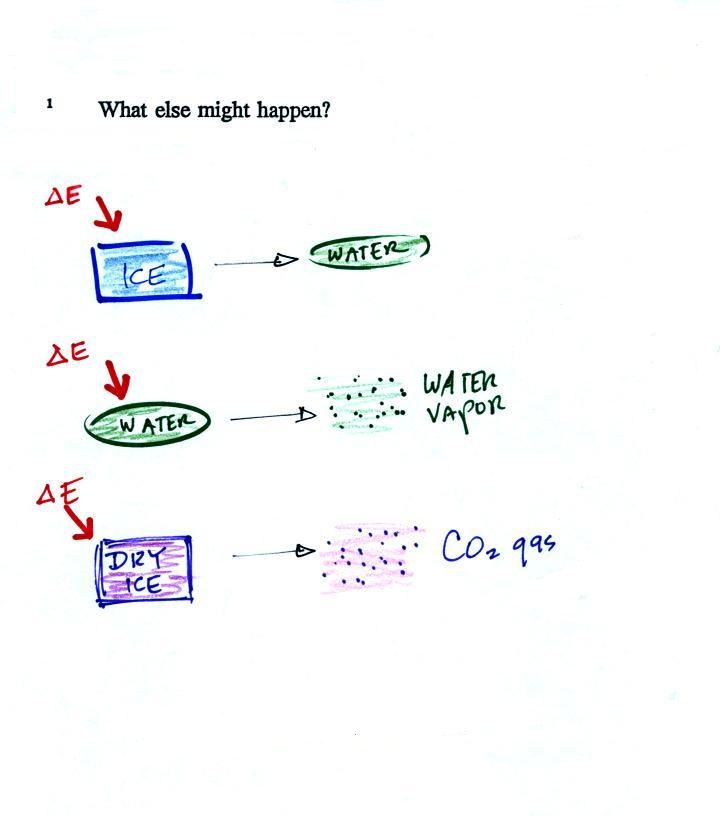
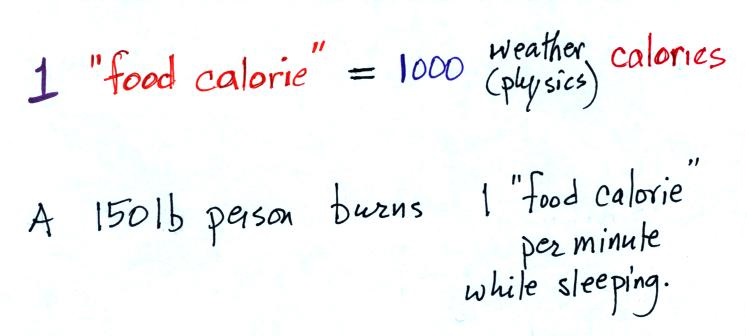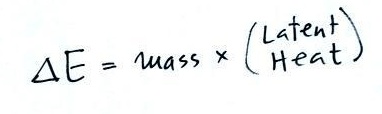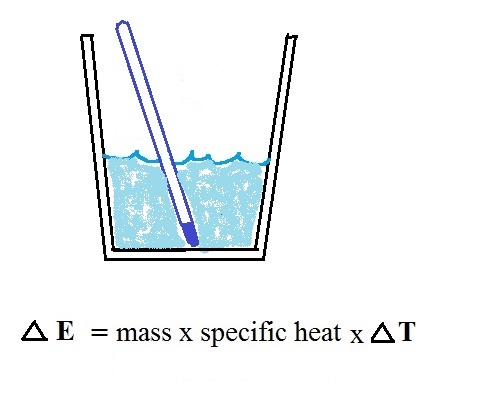Latent heat energy is an under-appreciated and
rather confusing type of energy. The word latent refers to
energy that is hidden. That's part of the
problem. But it is also the fact that the energy is
contained in water vapor and water. That seems like
an unlikely place for energy to be
found. The hidden energy emerges when
water vapor condenses or water freezes.
In the bottom picture above, sunlight shining on a
tropical ocean warms and evaporates ocean water. The
sunlight energy is stored in the resulting water
vapor. A hurricane derives much of its energy from
the condensation of water vapor (it also gets heat energy
from the warm ocean water).
Energy units
Now just brief mention of units of energy
Joules are the
units of energy that you would probably encounter in a
physics class. We'll usually be using calories
as units of energy. 1 calorie is the energy need
to warm 1 gram of water 1 C (there are about 5 grams
of water in a teaspoon). Your electric bill
shows the amount of energy that you have used in a
month's time, the units are kilowatt-hours.
Here's a little miscellaneous information that you
don't need to worry about remembering. You've
probably seen the caloric content of food on food packages
or on menus in restaurants. 1 food calorie is
actually 1000 of the calories mentioned above.
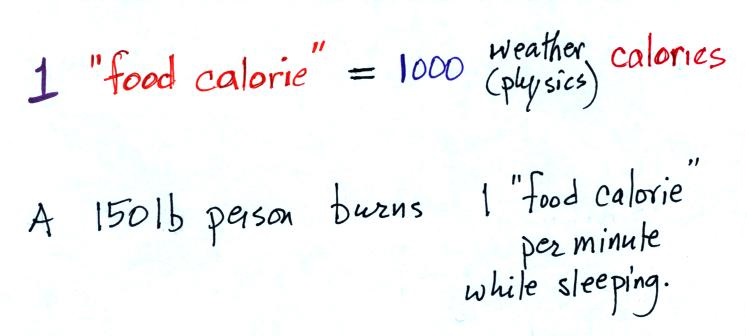
A 150 pound person would burn
almost 500 calories while sleeping during the night
(8 hours x 60 minutes per hour x 1 food calorie per
minute). This is about the energy contained in
a donut.
2. Energy transport processes
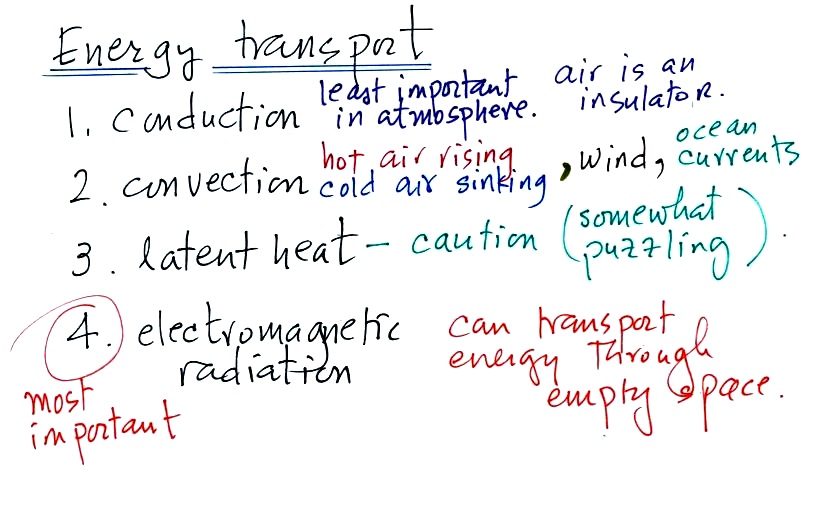
By far the most important process is at the
bottom of the list above. Energy transport in the form
of electromagnetic radiation (sunlight for example) is the
only process that can transport energy through empty
space. Electromagnetic radiation travels both to the
earth (from the sun) and away from the earth back into
space. Electromagnetic radiation is also responsible for
about 80% of the energy transported between the
ground and atmosphere.
You might be surprised to learn that latent heat is the
second most important transport process. This term
latent heat can refer to both a type of energy and an energy
transport process.
Rising parcels of warm air and sinking parcels of cold air
are examples of free convection. Because of convection
you feel colder or a cold windy day than on a cold calm day
(the wind chill effect). Ocean currents are
also an example of convection.
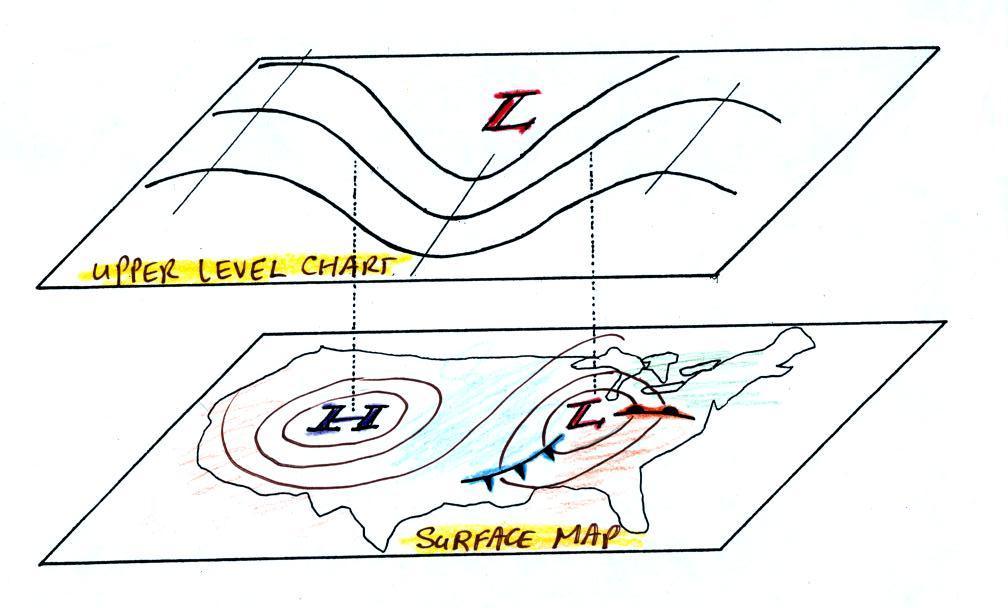
Convection is also one of the ways of rising air motions in
the atmosphere (convergence into centers of low pressure and
fronts are two other ways we've encountered so far)
Conduction is the least important energy transport at least
in the atmosphere. Air is such a poor conductor of
energy that it is generally considered to be an insulator.
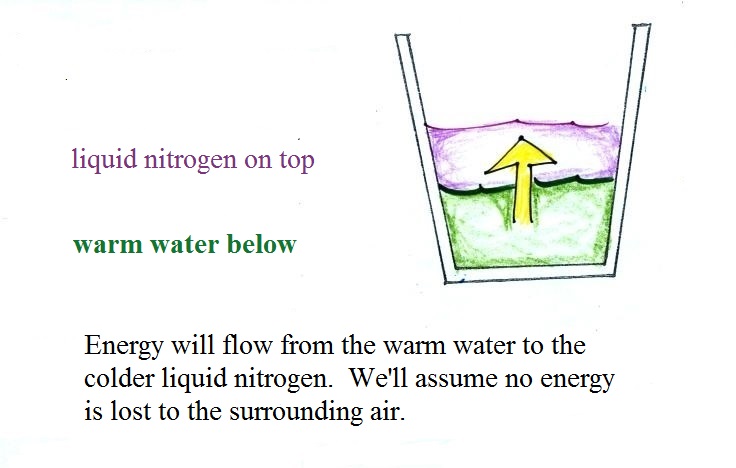
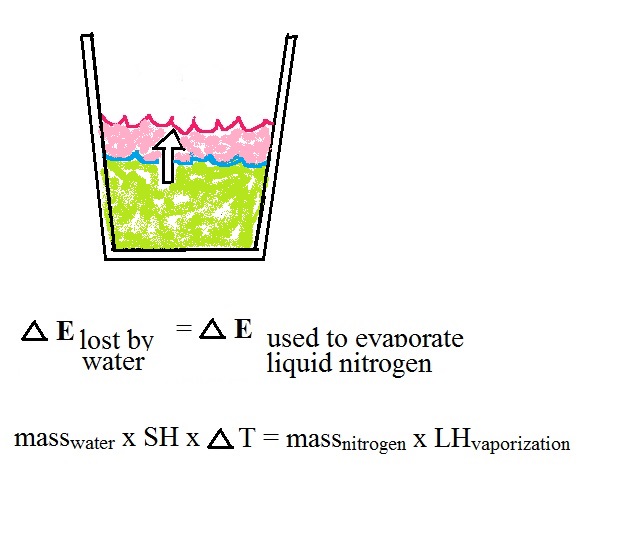
Energy will naturally flow from hot (the
water) to cold (the liquid nitrogen). As energy is taken
from the water it will cool. We'll assume that all of
the energy taken from the water is used to evaporate nitrogen,
no energy flows from the cup into the surrounding air (that's
part of the reason we conduct the experiment in a Styrofoam
cup.
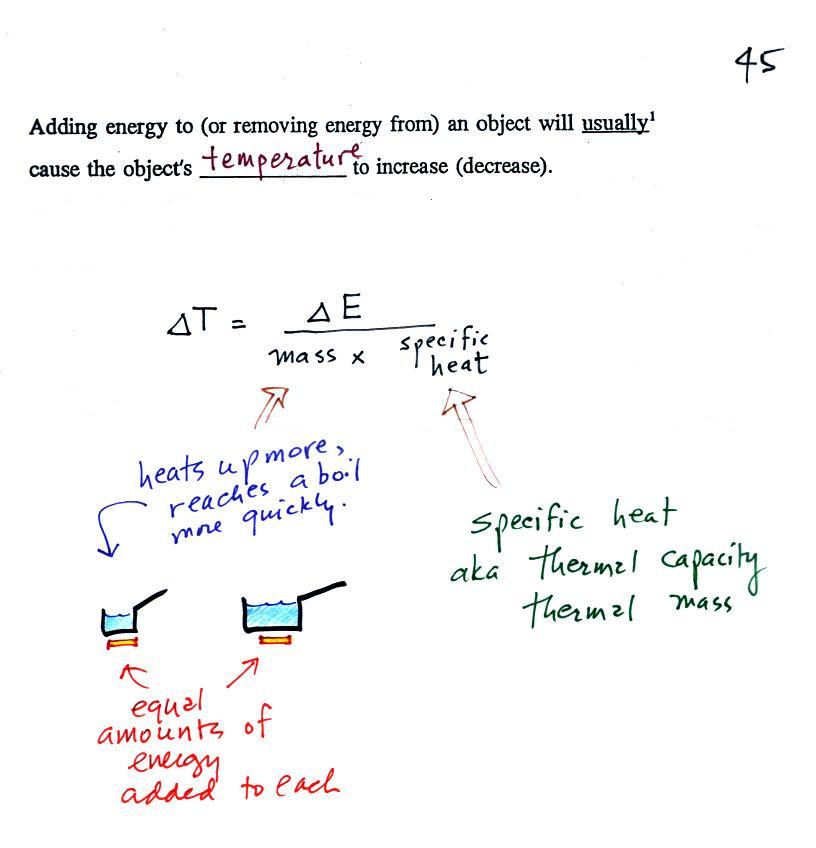
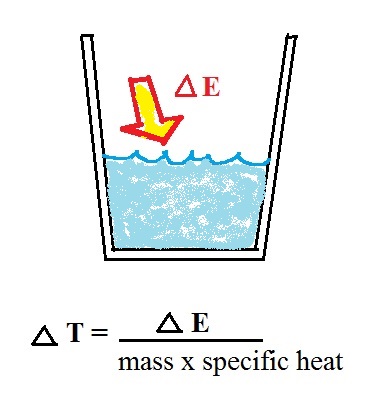
Our earlier equations is shown above at
left. If you know how much energy is added to something
you could determine the temperature change that would
result. We can turn the equation around so that is we
measure the temperature change that any object undergoes we
can calculate the amount of energy added or removed (the
equation at right).
Here we put everything together. We'll
determine how much energy is taken from the water.
Then we'll assume all of that energy is used to evaporate
nitrogen. That's the right hand equation above.
We start with a large
styrofoam cup filled about 1/3 full with room temperature
water.
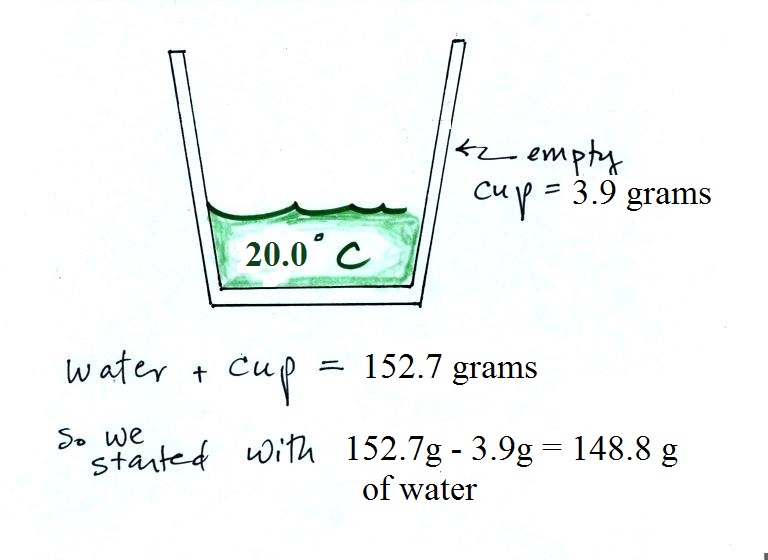
The cup and the water together weighed 152.7 g of room
temperature water. The cup weighed 3.9 g, so we really
had 148.8 g of water. The student measured its
temperature, 20.0 C.
Next the student poured some liquid nitrogen into a
second, smaller styrofoam cup.
We're going to evaporate 35 grams of liquid
nitrogen. The total amount of energy needed to do that,
ΔE, is the mass of the liquid nitrogen times the Latent Heat
of
vaporization of Nitrogen (LHvap).
ΔE = mass x LHvap
LHvap is the energy needed per gram to vaporize
(evaporate) liquid nitrogen. That's the quantity we are
trying to measure.
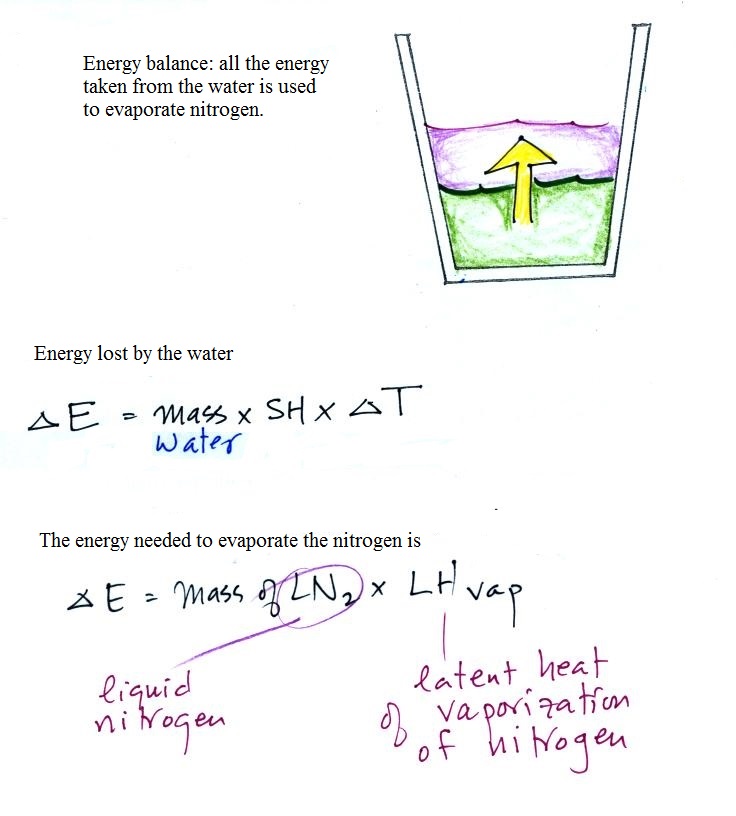
We poured the 35 grams of liquid nitrogen
into the cup containing 148.8 g of water. Energy flows
naturally from hot to cold. We assume that any energy
lost by the water is used to evaporate nitrogen.
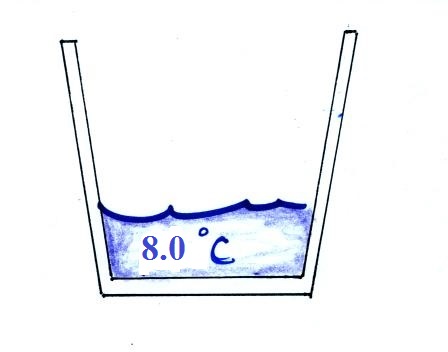
Once the liquid nitrogen was gone (it had
evaporated) we remeasured the water temperature. It had
dropped to 8.0 C. Now we're ready to
calculate the latent heat of vaporization
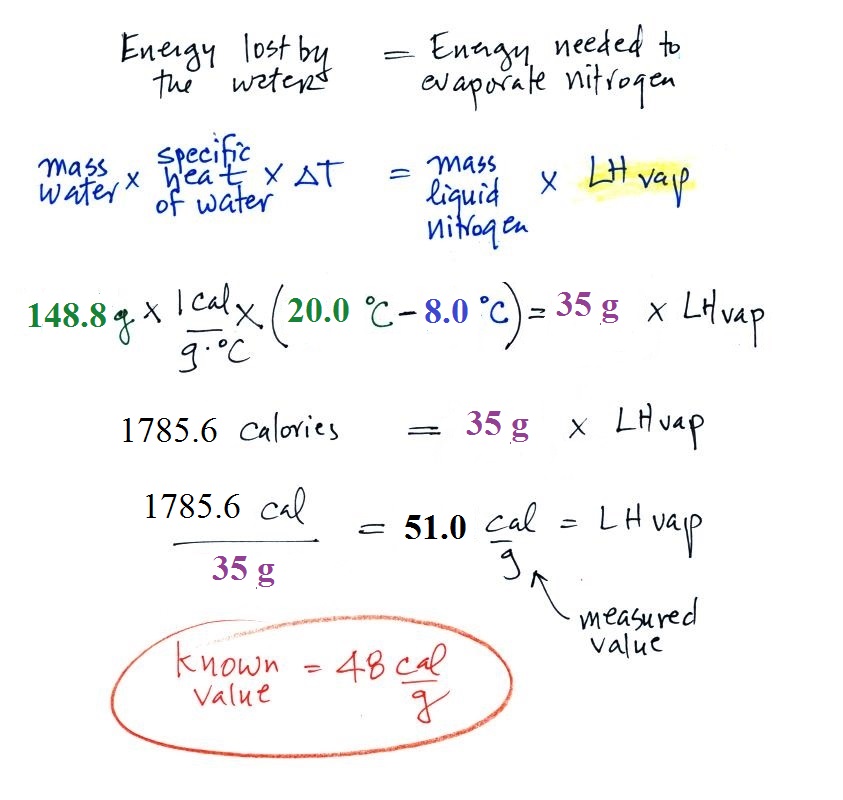
We set up an energy balance equation
(energy lost by the water = energy used to evaporate nitrogen)
and plugged in all our measured values. We obtained a
measured value of LHvap = 51.0 calories/gram (52.1 cal/g later
in the day in the 9:30 am class). A trustworthy student
in the class informed us that the known value is 48
cal/g. Not a bad result at all.
Here's a very little bit of more material. We'll go over
it quickly at the start of class on Tuesday, especially the
information on temperature scales next Tuesday. None of this was covered in class today.
Temperature and heat
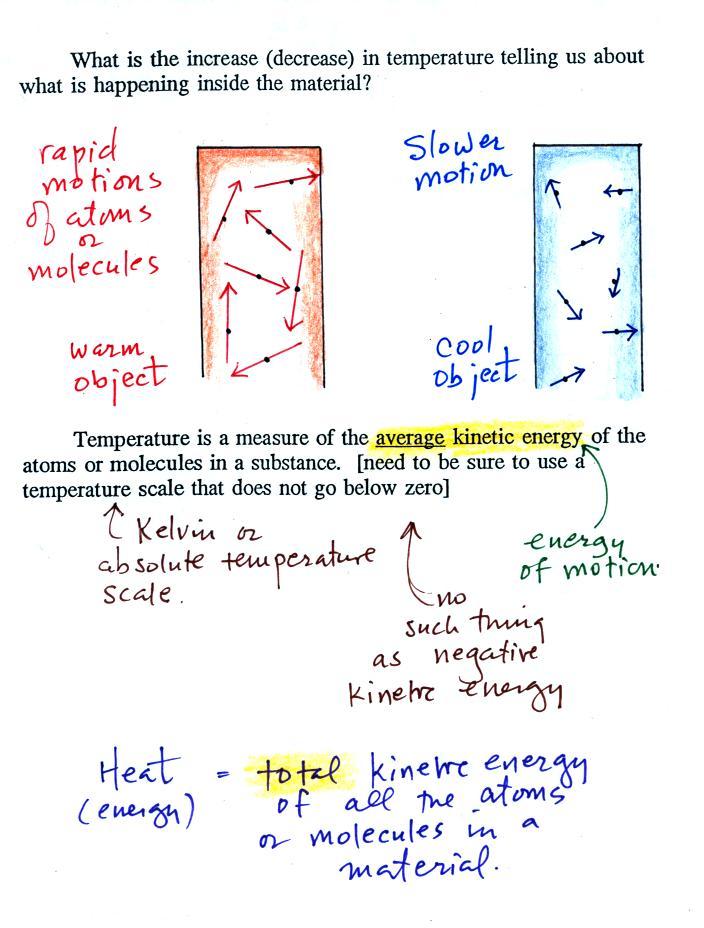
You add energy to something and its
temperature usually increases. The figure below (p. 46
in the ClassNotes) shows you what happens inside an object
when it's temperature changes.
The atoms or molecules inside
the warmer object will be moving more rapidly (they'll be
moving freely in a gas, just "jiggling" around while still
bonded to each other in a solid). Temperature
provides a measure of the average kinetic energy of the atoms
or molecules in a material.
You need to be careful what temperature scale you use when
using temperature as a measure of average kinetic
energy. You must use the Kelvin temperature scale
because it does not go below zero (0 K is known as absolute
zero). The smallest kinetic energy you can have is zero
kinetic energy. There is no such thing as negative
kinetic energy.
You can think of heat as being the total
kinetic energy of all the molecules or atoms in a material.
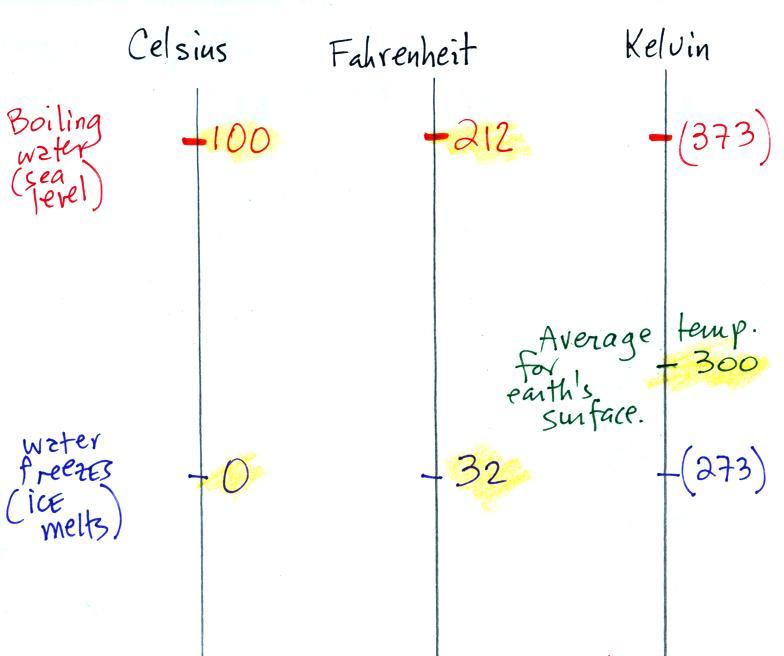
Temperature scales
Speaking of
temperature scales
You should remember the temperatures of the
boiling point and freezing point of water on at least the
Fahrenheit and Celsius scales (and the Kelvin scale if
you want to). 300 K is a good easy-to-remember value for
the global annual average surface temperature of the
earth. Remember 300 K and also that temperature never
goes below zero on the Kelvin scale.
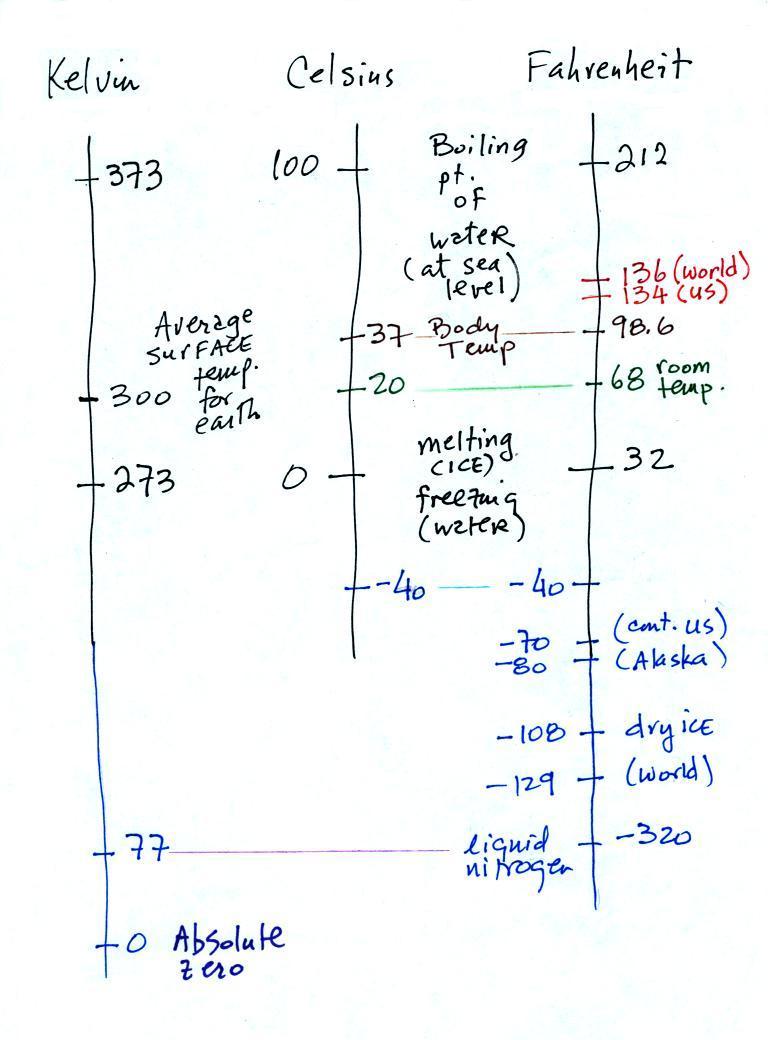
You certainly don't need to try to remember
all these numbers. The world high temperature record
value of 136 F above was measured in Libya at a location that
was only about 35 miles from the Mediterranean coast.
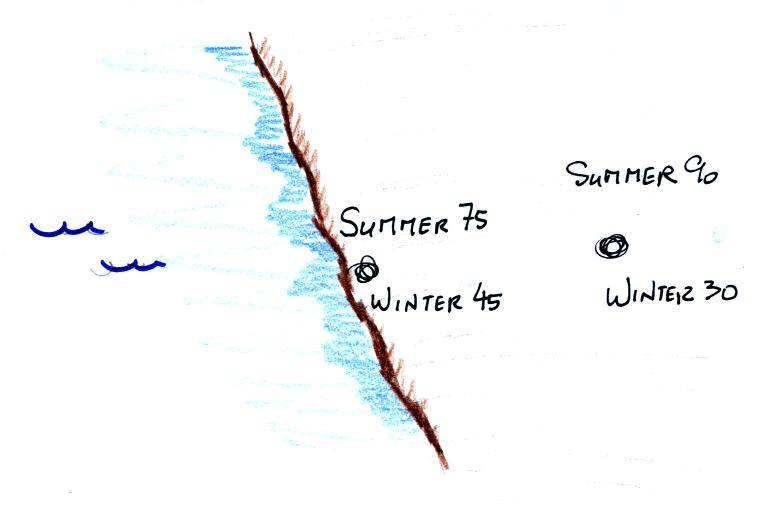
Water, as we have seen, moderates climate so it seemed odd
that such a high temperature would have been recorded
there. The World Meteorological Organization recently
decided the 136 F reading was invalid and the new world record
is the 134 F measurement made in Death Valley.
The continental US cold temperature record of -70 F was
set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in
Antarctica. This unusually cold reading was the result
of three factors: high latitude, high altitude, and location
in the middle of land rather than being near or surrounded by
ocean (again water moderates climate, both hot and
cold).
Liquid nitrogen is very cold but it is still quite a bit
warmer than absolute zero. Liquid helium gets within a
few degrees of absolute zero, but it's expensive and there's
only a limited amount of helium available. So I would
feel guilty bringing some to class and I don't think it would
look any different than liquid nitrogen.