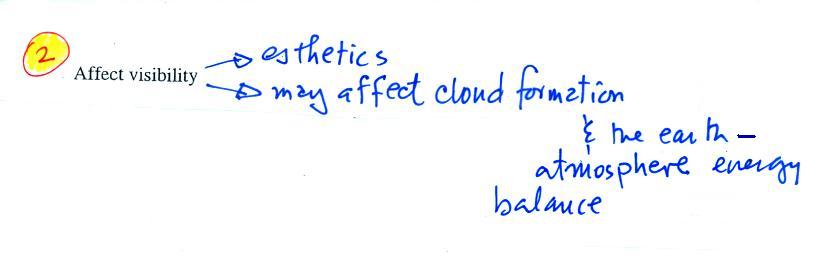Tuesday, Jan. 27, 2015
Lost Bayou Ramblers "Four
Leaf Clover", "Blue
Moon Special", "My Generation
(in French)" and "Moi j'connais
pas".
National Ambient Air Quality
Standards (NAAQS) and the Air Quality Index (AQI)
We started by quickly going over some material
that was stuck onto the end of the Thursday
Jan. 22 lecture notes.
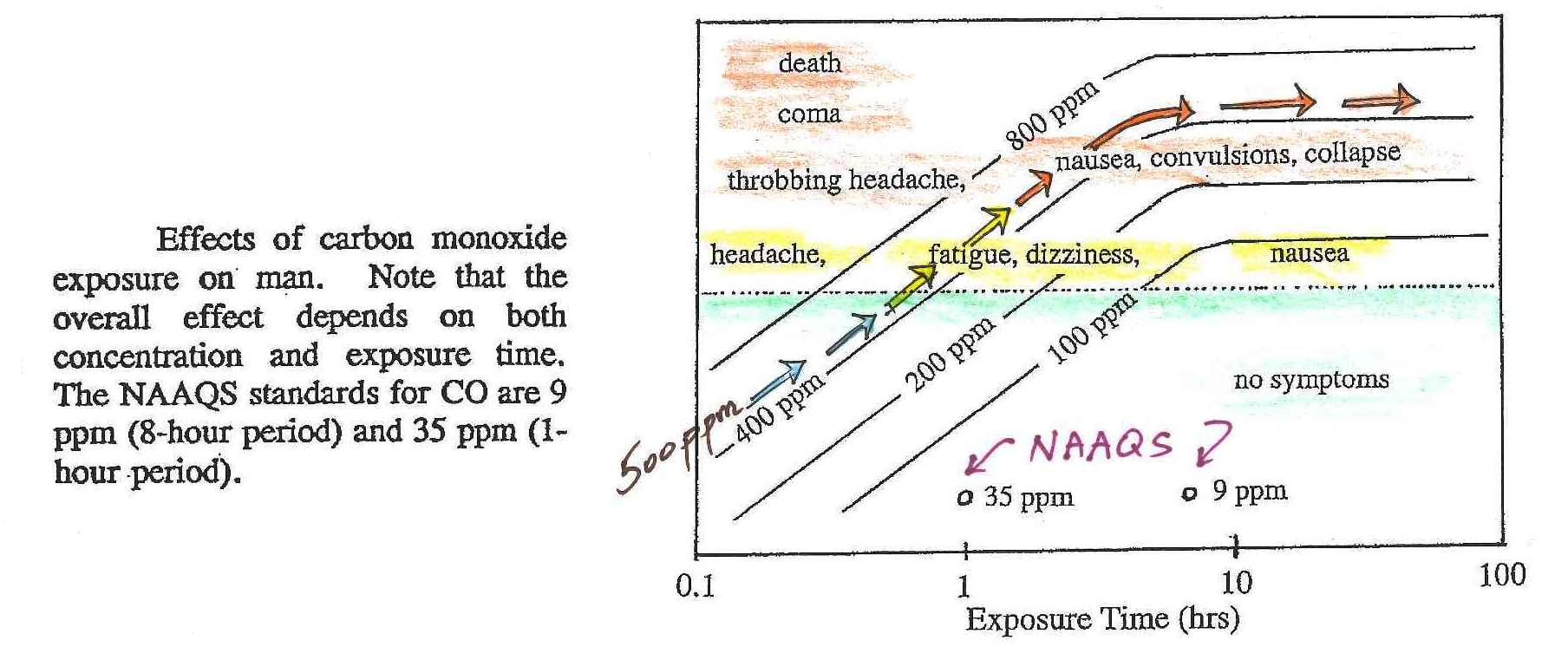
Symptoms of carbon monoxide poisoning
Carbon monoxide is a
serious hazard indoors where it can build to much
higher levels than would ever be found outdoors.
This next link is to a newspaper article describing an
incident at Virginia Tech (that occurred near the
beginning of the school year in 2007). Carbon
monoxide
from
a malfunctioning hot water heater sickened 23 Virginia
Tech students in an apartment complex. The CO
concentration is thought to have reached 500 ppm.
You can get an
idea of what kinds of symptoms and effects that concentrations
this high could cause from the figure in the middle of p. 9 in
the photocopied ClassNotes.
The effects of CO depend on what concentration you exposed to
and the duration of the exposure. In this case we'll
follow the arrows from lower left to the upper right of the
figure. The arrows represent a concentration of about 500
ppm. Beginning at lower left we see that we wouldn't
experience any symptoms with an exposure to even 500 ppm for
just a few minutes. Note also the NAAQS values near the
bottom of the graph. Beginning at about 1 hour exposure
the arrows cross from the lower green half to the upper
yellow and orange half of the graph. Beginning at 1 hour
you would experience headache, fatigue, dizziness, nausea.
The symptoms would worsen if the exposure lasted for a few
hours: throbbing headache, nausea, convulsions, and
collapse. The 500 ppm line comes very close to coma and
death part of the graph. At Virginia
Tech several students were found unconscious and a few had
difficulty breathing on their own but were resuscitated; they
very nearly died.
Carbon monoxide alarms are relatively inexpensive
(~$50) and are available at most hardware stores. I've got
one in my house to protect me and my cats. They will
monitor CO concentrations indoors and warn you when
concentrations reach hazardous levels.
Indoors CO is produced by gas furnaces and water heaters that
are either operating improperly or aren't being properly vented
to the outdoors. A few hundred people are killed indoors
by carbon monoxide every year in the United States. An
operating carbon monoxide alarm probably saved the lives of the
6 Tucson residents in December 2010. You can learn
more about carbon monoxide hazards and risk prevention at the Consumer
Product Safety Commission web page.
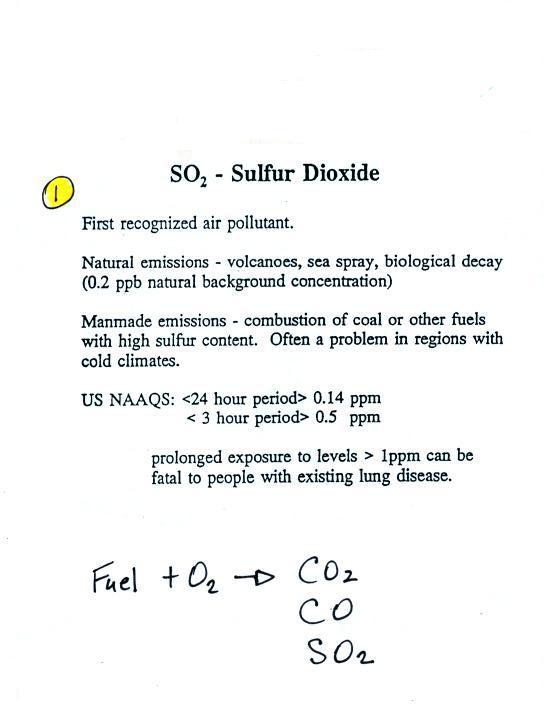
Sulfur dioxide (SO2 )
We turn now to the 3rd of the air
pollutants we will cover, sulfur
dioxide (SO2 ).
Sulfur dioxide is produced by the combustion of sulfur
containing fuels such as coal. Combustion of fuel also
produces carbon dioxide and carbon monoxide. People
probably first became aware of sulfur dioxide because it has an
unpleasant smell. Carbon dioxide and carbon monoxide are
odorless. That is most likely why sulfur dioxide was the
first pollutant people became aware of.
I've checked on the smell of sulfur dioxide class and have found
two descriptions: one described it as the smell of rotten eggs
(I associate that with hydrogen
sulfide, H2S, which is also
poisonous), the second is a pungent irritating odor which is
what I remember. Apparently sulfur dioxide is one of the
smells in a freshly struck match.
Volcanoes are a natural source of sulfur dioxide.
London-type smog

The inversion layer in this case lasted for several days and
was produced in a different way than the surface radiation
inversions we heard about when covering carbon monoxide.
Surface radiation inversions usually only last for a few
hours.
The term smog, a contraction of smoke + fog, was invented to
describe a mixture of smoke and fog, something that was fairly
common in the winter in London. The 1952 event was an
extreme case. Now we distinguish between "London-type
smog" which contains sulfur dioxide and photochemical or "Los
Angeles-type smog" which contains ozone.
Most
of the photographs below come
from articles published in 2002 and 2012, the
50th and 60th anniversaries of the event.
The caption to this
photo from The Guardian reads
"Arsenal goalkeeper Jack Kelsey peers into the
fog.
The 'smog' was so thick the game was eventually
stopped."
|

This is about the thickest smog I was able to
find. Visibility here is perhaps 10 or 20 feet.
(source
of this image)
|
|
|
|
Smog masks from this
reference
The masks would filter out the smoke but not the
sulfur dioxide gas
|
Here are some interesting photographs
of early and mid 20th century London.
The sulfur dioxide didn't
kill people directly. Rather it
would aggravate an existing condition of some
kind. The SO2 probably also
made people susceptible to bacterial infections
such as pneumonia. Here's
a link that discusses the event
and its health effects in more detail.
The Clean
Air Act of 1956 in England reduced smoke
pollution and emissions of sulfur dioxide.
However an
article in The Telegraph notes that London air
now exceeds recommended concentration limits for
nitrogen dioxide and particulates.
Air
pollution disasters involving sulfur dioxide have also
occurred in the US. One of the deadliest events was in
1948 in Donora, Pennsylvania.
The reference
material that contained this photographed clearly
stated "This eerie photograph was taken at noon on
Oct. 29, 1948 in Donora, PA as deadly smog enveloped the
town. 20 people were asphyxiated and more than 7,000
became seriously ill during this horrible event."
The photograph below shows some of the mills that were operating
in Donora at the time. Not only where the factories adding
pollutants to the air they were undoubtedly adding hazardous
chemicals to the water in the nearby river.
from: http://www.eoearth.org/article/Donora,
Pennsylvania
The US passed its own
Clean Air Act in 1963. There have been several
major revisions since then. The EPA began
in late 1970 (following an executive order signed by President
Nixon)
"When Smoke Ran Like Water,"
a book about air pollution is among the books
that you can check out, read, and report on to
fulfill part of the writing requirements in this
class (though I would encourage you to do an
experiment instead). The author, Devra
Davis, lived in Donora Pennsylvania at the time
of the 1948 air pollution episode.
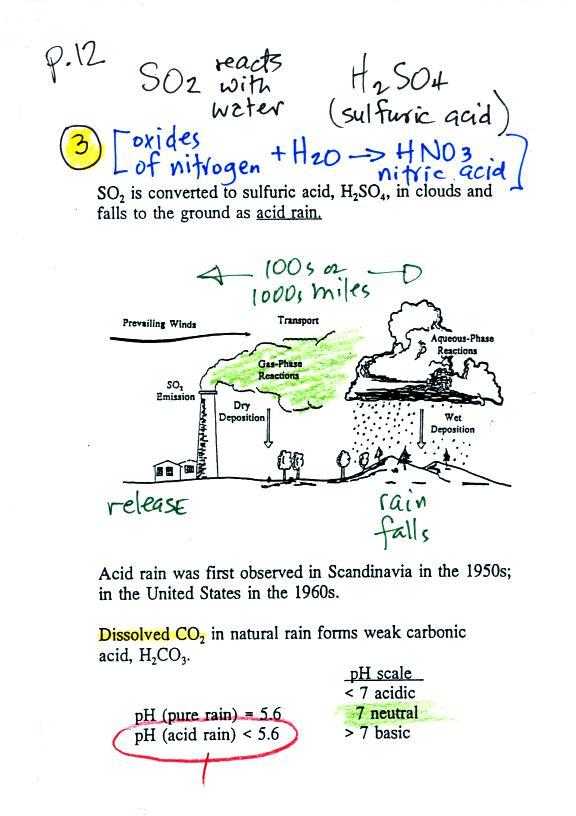
Acid rain
Sulfur
dioxide is one of the pollutants that can react with
water in clouds to form acid rain (some of the oxides
of nitrogen can also react with water to form nitric
acid). The formation and effects of acid rain
are discussed on p. 12 in the photocopied Class Notes.
Acid rain is often a problem in regions that are 100s even
1000s of miles from the source of the sulfur dioxide. Acid
rain in Canada could come from sources in the US, acid rain in
Scandinavia came from industrialized areas in other parts of
Europe.
Note at the bottom of the figure above that natural "pristine"
rain has a pH less than 7 and is slightly acidic. This is
because the rain contains dissolved carbon dioxide gas. The
acid rain demonstration described below and done in class should
make this point clearer.
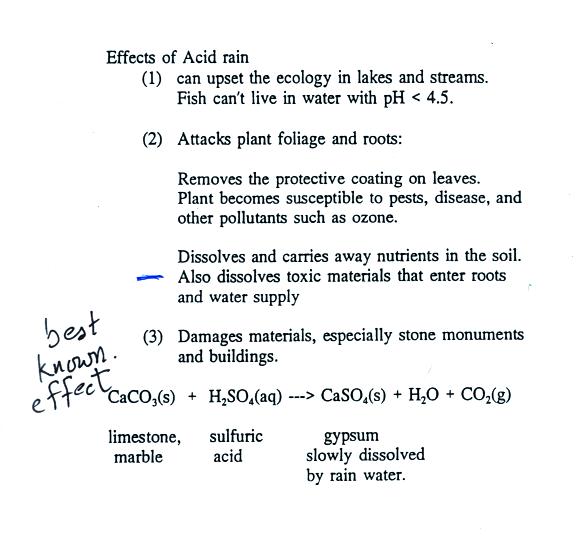
Some of the problems associated with acid rain are listed above.
Click on this acid
rain demonstration link for a detailed description of the
demonstration done in class.
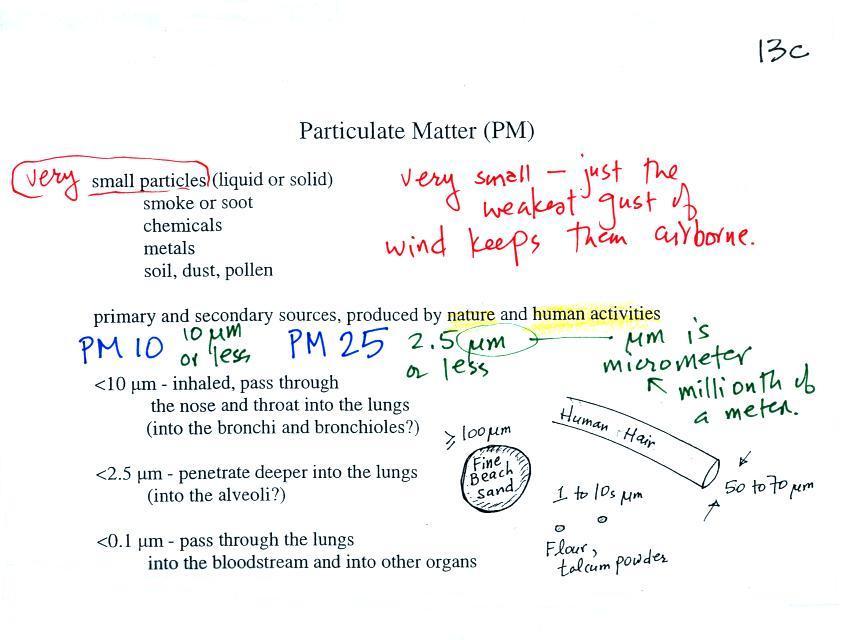
Particulate matter
The last pollutant that we will cover is Particulate Matter
(PM) - small solid particles or drops of liquid (but not gas) that
remain suspended in the air (particulates are sometimes referred
to as aerosols).
The designations PM10 and PM2.5 refer to particles with diameters
less than 10 micrometers and 2.5 micrometers, respectively.
A micrometer (µm) is one millionth of a meter (10-6 m). You'll find
some actual pictures of micrometer sized objects and more
information at this
interesting site. Red blood cells are 6-10 µm
in diameter. A nanometer (nm) is 1000 times
smaller than a micrometer (10-9
m). An atom is apparently 0.1 to 0.3 nm across, depending on
the particular element.
Particulate matter can be
produced naturally (wind blown dust, clouds above volcanic
eruptions, smoke from lightning-caused forest and brush
fires). Human activities also produce particulates.
Gases sometimes react in the atmosphere to make small drops or
particles (this is what happened in the photochemical smog
demonstration). Just the smallest, weakest gust of wind is
enough to keep particles this small suspended in the atmosphere.
One of the main concerns with particulate pollution is
that the small particles might be a health hazard ( a health
advisory is sometimes issued during windy and dusty conditions in
Tucson)
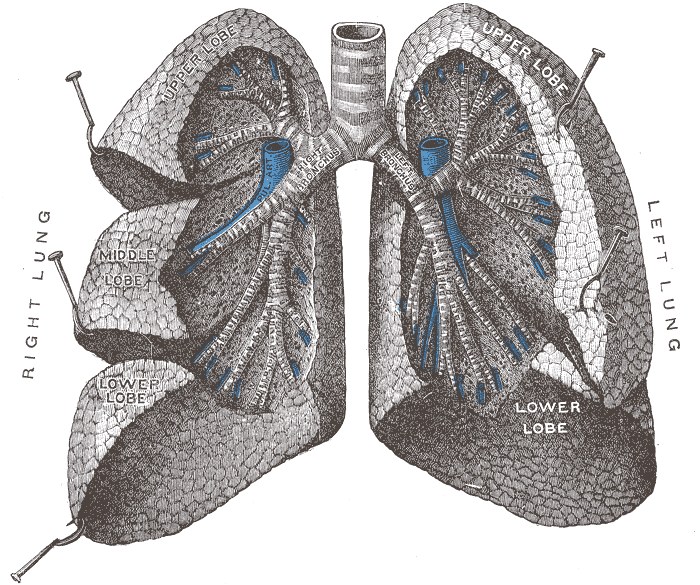
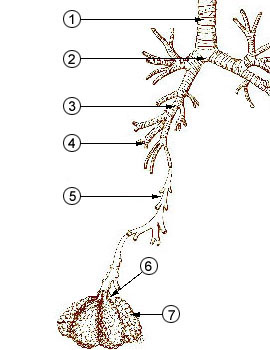
Particles with dimensions of 10 µm
and less can be inhaled into the lungs (larger particles get
caught in the nasal passages). These inhaled particles
may be poisonous, might cause cancer, damage lung tissue, or
aggravate existing respiratory diseases. The smallest
particles can pass through the lungs and get into the blood
stream (just as oxygen does) and damage other organs in the
body.
The figure below identifies some of the parts of the human
lung mentioned above.
Note the PM10 annual
National Ambient Air Quality Standard (NAAQS) value of
50 micrograms/cubic meter (µg/m3)
at the bottom of p. 13c in the photocopied
ClassNotes.
The following list (p. 13d in the ClassNotes) shows that there
are several cities around the world where PM concentrations are 2
or 3 times higher than the NAAQS value.
The World Health Organization recommends that PM2.5 concentrations
be kept below 25 µg/m3.
Particulate concentrations during an air pollution event in
Beijing in 2013 apparently reached several hundred µg/m3
at the US Embassy. Someone mentioned fireworks in
class today. The large fireworks displays that
sometimes occur in Beijing do produce a lot of particulate
pollution (reference).
Fireworks are illegal in Tucson because of the risk of
wildfires.
The 2008 Summer Olympics were held in Beijing and there
was some concern that the polluted air would affect the
athletes performance. Chinese authorities restricted
transportation and industrial activities before and during
the games in an attempt to reduce pollutant
concentrations. Rainy weather during the games may
have done the greatest amount of good.
Clouds and precipitation are the best way of cleaning
pollutants from the air. We'll learn later in the semester
that cloud droplets form on small particles in the air called
condensation nuclei. The cloud droplets then form raindrops
and fall to the ground carrying the particles with them.
The second main concern with particulates is the
effect they may have on visibility (esthetics below should
actually be spelled aesthetics - i.e. qualities that might
make something appear beautiful or not).
Here's a view of the Catalina mountains taken from the Gould
Simpson Building on the south side of campus.
Some rainy weather had occurred just a day to two earlier, cleaned
the air, and the visibility was very good.
Windy weather a few days later
stirred up a lot of dust that was carried into town.
This picture was taken the day after the windy weather.
There is still a lot of fine dust particles in the air and the
visibility is pretty bad.
We looked at some photographs from Beijing
(January, 2013) last week. Here are some pictures from Harbin,
China (October, 2013). That's about as bad as it can get,
visibility in some cases is just a few 10s of feet. Also a
picture from Paris
(March, 2014).