Friday, Apr. 1, 2016
Copenhagen Philharmonic Symphony "Ravel's
Bolero" (4:52), Grieg's "Peer Gynt"
(2:16), Naturally 7 performing Phil Collin's "In the Air
Tonight" (5:12)
Precipitation producing processes
The last topic we will cover before
next week's quiz is precipitation formation and types of
precipitation.
Only two of the 10 main cloud types (nimbostratus and
cumulonimbus) are able to produce significant amounts of
precipitation and produce precipitation that can survive
the fall from cloud to ground without evaporating. Why
is that?
Before we get into the details you will notice I underlined
significant amounts in the sentence above. That is because
you will sometimes see streamers of precipitation falling from
some of the other cloud types, clouds that you would not have
thought capable of producing precipitation. There were a few
raindrops falling from stratocumulus clouds after the 11 am class
last Wednesday. I've included some other examples below

|

|
Streamers of snow falling from either
mid or high altitude clouds at sunset. (source
of this image)
|
Snow falling from high altitude cirrus
uncinus clouds, photographed in Catalina, Arizona, I
believe. (source
of this image)
|
|
Precipitation like this that evaporates (or sublimes) before
reaching the ground is called virga. The rain that was
coming from low altitude clouds on Wednesday this week survived
the fall to the ground, but it was very light. There wasn't
enough to even dampen the ground.
Why is it so hard for clouds to make precipitation?
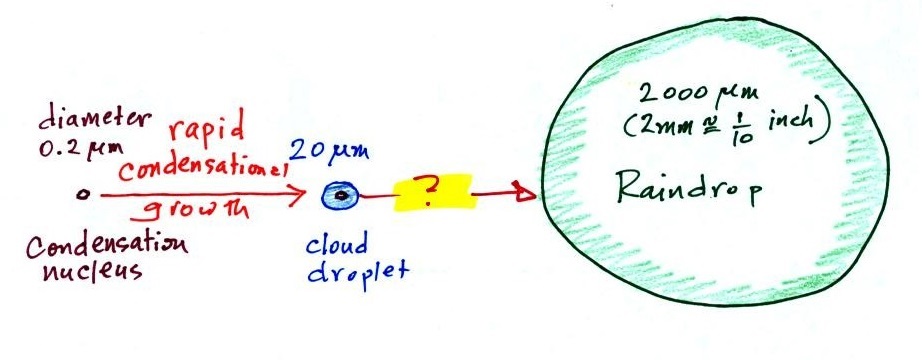
This figure shows typical sizes of cloud condensation
nuclei (CCN), cloud droplets, and raindrops (a human hair
is about 50 μm thick for comparison).
As we saw in the cloud in a bottle demonstration it is relatively
easy to make cloud droplets. You cool moist air to the dew
point and raise the RH to 100%. Water vapor condenses pretty
much instantaneously onto a cloud condensation nucleus to form a
cloud droplet. It would take much longer (a day or more) for
condensation to turn a cloud droplet into a raindrop. You
must know from personal experience that once a cloud forms you
don't have to wait that long for precipitation to begin to fall.
Part of the problem is that it takes quite a few 20 μm
diameter cloud droplets to make one 2000 μm
diameter raindrop. A raindrop is about 100 times bigger
across than a cloud droplet. How many droplets are needed to
make a raindrop? Before answering that question we will look
at a cube (rather than a sphere).
How many sugar cubes would
you need to make a box that is 4 sugar cubes on a side?
It
would take 16 sugar cubes to make each layer and
there are 4 layers. So you'd need 64 sugar
cubes. Volume is length x width x height.
The raindrop is 100 times wider, 100 times
bigger from front to back, and 100 times taller than
the cloud droplet. The raindrop has a volume
that is 100 x 100 x 100 = 1,000,000 (one million)
times larger than the volume of the cloud
droplets. It takes about a million
cloud droplets to make one average size raindrop.
Precipitation-producing
processes
Fortunately there are two processes capable of quickly turning
small cloud droplets into much larger precipitation particles
in a cloud.
The collision coalescence process works in clouds that are
composed of water droplets only. This is often called the
"warm rain" process. Clouds like this are only found in
the tropics. We'll see that this is a pretty easy process
to understand.

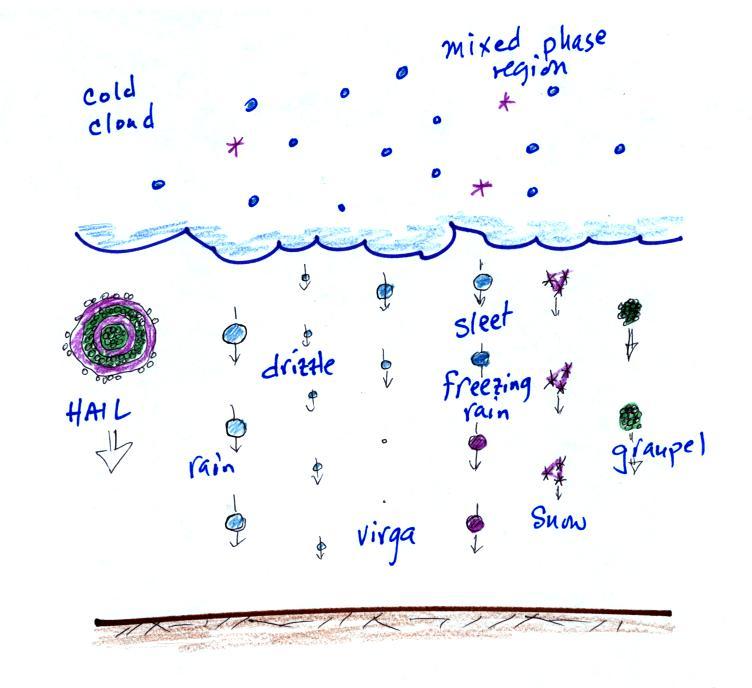
This process will only produce rain, drizzle, and something
called virga (rain that evaporates before reaching the
ground). Because the clouds are warm and warm air can
potentially contain more water vapor than cooler air, the
collision-coalescence process can produce very large amounts of
rain.
The ice crystal process produces precipitation everywhere
else. This is the process that makes rain in Tucson, even on
the hottest day in the summer (summer thunderstorm clouds are tall
and reach into cold parts of the atmosphere, well below
freezing). Hail and graupel
often fall from these storms; proof that the precipitation started
out as an ice particle). Thunderstorms also produce
lightning and later in the semester we will find that ice is needed to make the electrical charge
that leads to lightning.
There is one part of this process that is a
little harder to understand, but look at the variety of
different kinds of precipitation particles (rain, snow,
hail, sleet, graupel, etc) that can result.
Here's how the collision coalescence process works. The
picture below shows what you might see if you looked
inside a warm cloud with just water droplets:
The collision coalescence
process works in a cloud filled with cloud droplets of
different sizes, that's critical. The
larger droplets fall faster than the small droplets. A
larger-than-average cloud droplet will overtake and collide with
smaller slower moving ones.
The bigger droplets
fall faster than the slower ones. They collide and
stick together (coalesce). The big drops gets even
bigger, fall faster, and collide more often with the smaller
droplets. This is an accelerating growth process -
think of a growing ball of snow as it rolls down a
snow-covered hill and picks up snow, grows, and starts to
roll faster and faster; or think of an avalanche
that gets bigger and moves faster as it travels downslope.
A larger than average cloud droplet can very quickly grow
to raindrop size.
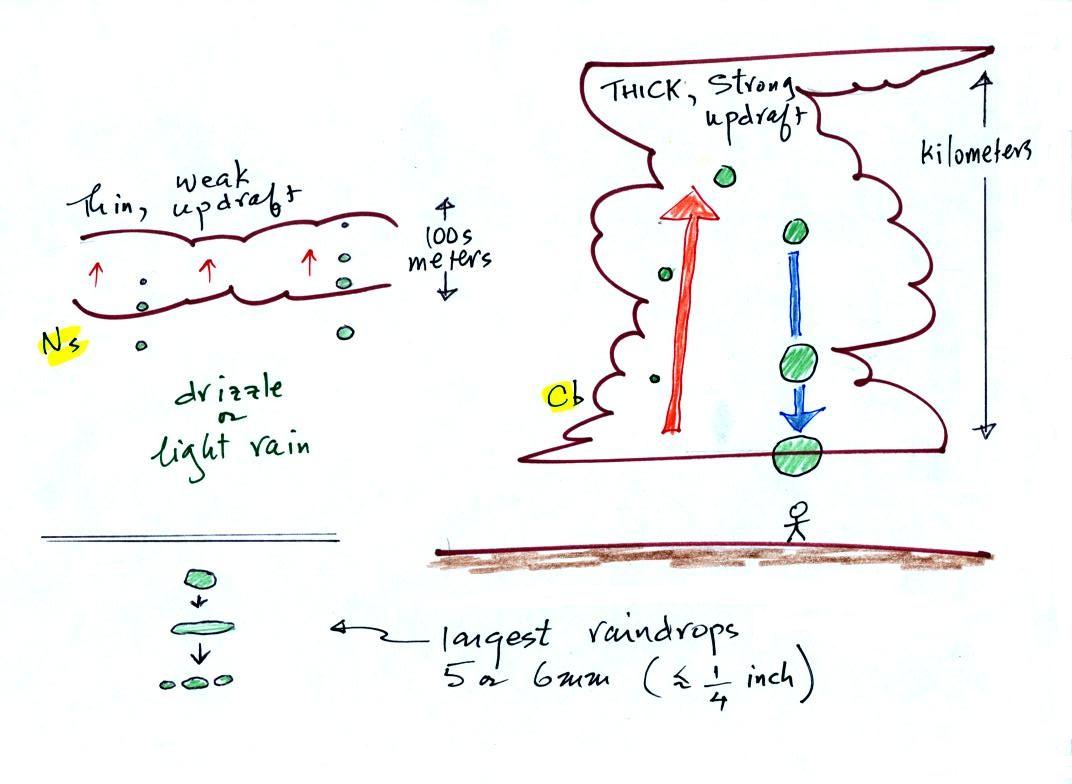
The figure shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns
clouds are thinner and have weaker updrafts than Cb clouds.
The largest raindrops fall from Cb clouds because the droplets
spend more time in the cloud growing. In a Cb cloud raindrops can
grow while being carried upward by the updraft and also when
falling in the downdraft.
Raindrops grow up to about 1/4 inch in diameter. When
drops get larger than that, wind resistance flattens out the drop
as it falls toward the ground. The drop begins to "flop" or
"wobble" around and breaks apart into several smaller
droplets. Solid precipitation particles such as hail can get
much larger (an inch or two or three in diameter).
And actually my sketch at lower left above isn't quite accurate
as this video of the breakup of a
5 mm diameter drop of water shows.
The ice crystal process works in most locations most of the
time. Before we can look at how the ice crystal process
actually works we need to learn a little bit about clouds that
contain ice crystals - cold clouds.
Cold clouds
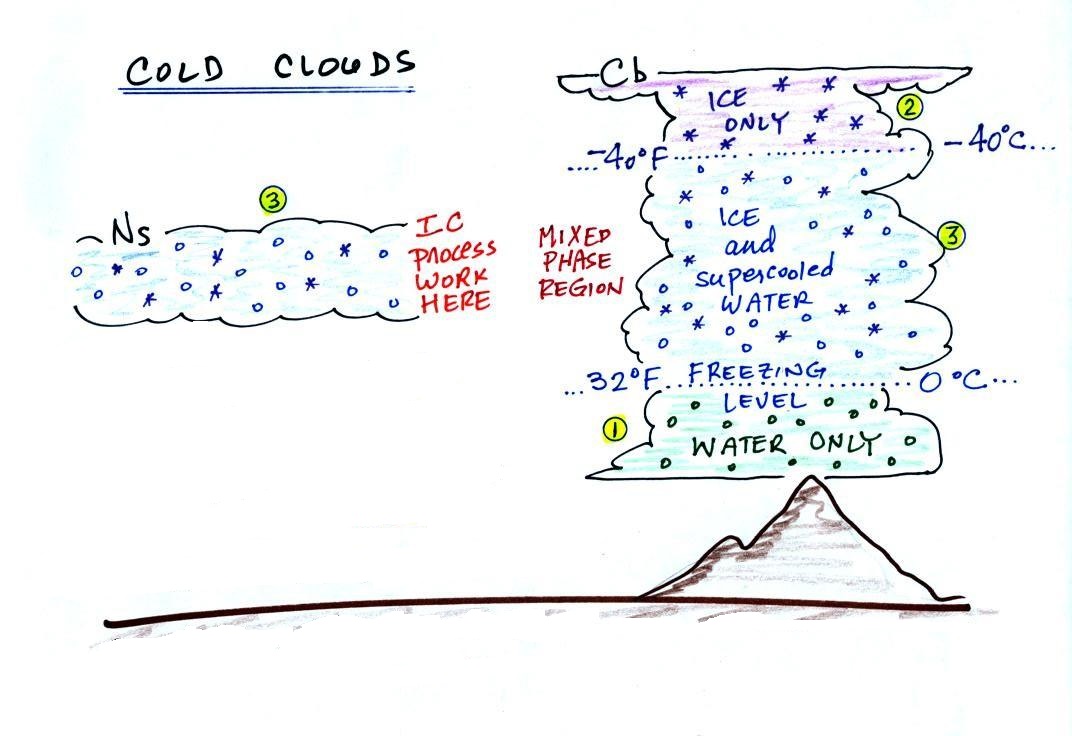
The figure below shows the interior of a
cold cloud.
The bottom of the thunderstorm, Point 1, is warm enough (warmer
than freezing) to just contain water droplets. The top of
the thunderstorm, Point 2, is colder than -40 F (which,
coincidentally, is equal to -40 C) and just contains ice
crystals. The interesting part of the thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains both
supercooled water droplets (water that has been cooled to below
freezing but hasn't frozen) and ice crystals. This is called
the mixed phase region.
This is where the ice crystal process will be able to produce
precipitation. This is also where the electrical charge that
results in lightning is created.
Ice crystal nuclei

The supercooled water droplets aren't able
to freeze even though they have been cooled below
freezing. This is because it is much easier for small
droplets of water to freeze onto an ice crystal nucleus (just
like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small
droplet of pure water). Not just any material will work
as an ice nucleus however. The material must have a
crystalline structure that is like that of ice. There
just aren't very many materials with this property and as a
result ice crystal nuclei are rather scarce. In most of
the mixed phase region there are more supercooled water
droplets than ice crystals.
Supercooled water
Here are a couple of demonstrations involving supercooled water
that I showed in class. In the first
demonstration, some supercooled water (cooled to -6 F (-21
C)) is poured into a glass bowl sitting at room
temperature. Just pouring the water into the bowl is
enough of a "disturbance" to cause the supercooled water to
freeze. Just bumping a bottle of supercooled water in the second
video is enough to cause the water to freeze. I
don't know why that happens.
Superheated water
It is also possible to superheat water.
When the superheated water is disturbed it
suddenly and boils explosively. This
is a potentially dangerous demonstration to attempt, better to
watch a
video online.
Here are a some precautions just in case you're ever tempted to
try an experiment like this.
It is probably easier to superheat distilled water than ordinary
tap water. So you might put two cups of water into a
microwave, one with tap water the other filled with distilled
water. The cup of tap water will probably start boiling when
it is supposed to, i.e. before it can become superheated.
You can watch the tap water and get an idea of how long you need
to heat the distilled water to superheat it. I suspect
impurities in the tap water might act as nuclei to initiate the
boiling.
Then once you think you have superheated the cup of distilled
water be very careful taking it out of the microwave (better yet
leave it in the microwave). Just the slightest disturbance
might start the water boiling. You want your hands, arm,
body and faced covered and protected just in case this
happens. Tape a spoon onto the end of a long stick and put a
little sugar or salt into the spoon. Then drop the salt or
sugar into the cup of superheated water.
Chemists will often use "boiling chips" to make sure water will
start to boil when it is supposed to (at 212 F) rather than
becoming superheated.
Bubbles in beer or soda
Rather than superheating water, here's a far safer
experiment to try.
Carbonated drinks all contain dissolved carbon dioxide. The
drink containers are pressurized. When you open the can or
take the cap off the pressure inside is released and dissolved
carbon dioxide gas starts to come out of solution and forms small
bubbles. Often you will see the bubbles originate at a point
on the side or bottom of the glass. These are nucleation
sites and are often small scratches or pits on the surface of the
glass that are filled with a small bubble of air. You can
think of these bubbles of air as being "bubble nuclei." When
the carbon dioxide comes out of solution rather than forming a
small bubble of its own, it makes use of and builds on these
existing bubbles of air. The bubble, now a mixture of air
and carbon dioxide, grows until it is able to break free and float
to the surface (a little gas is left behind in the scratch so the
process can start over again).

This is actually a michelada, I think; a
mixture of beer, lime, and tomato juice(image
source) but that doesn't affect the bubble formation
The next time you are drinking one of these carbonated beverages
sprinkle in a few grains of sugar or salt. These will serve
as additional bubble nucleation sites and additional bubbles will
form. This is exactly what happened in the superheated water
demonstration above.
This is as far as I dared go in class
today. I've moved the rest of the information about the
formation of precipitation and types of precipitation to the
Monday, Apr. 4 notes.