Latent heat energy transport involves
changes in phase or state. You should be able to
name each of these phase changes sketched above (this is
p. 55 in the ClassNotes). You should also be able to
indicate whether energy must be added to or removed from
the material in order for each phase change to take
place. For example, do you need to add energy to ice
or take energy from a piece of ice to cause it to melt.
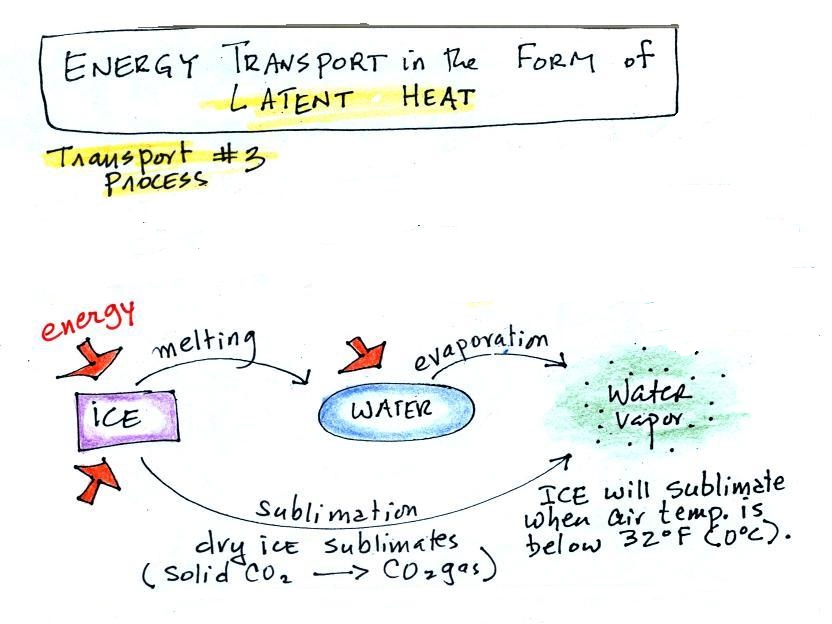
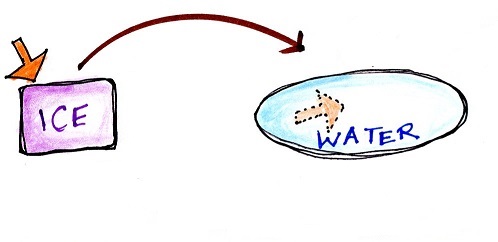
A solid to liquid phase change is
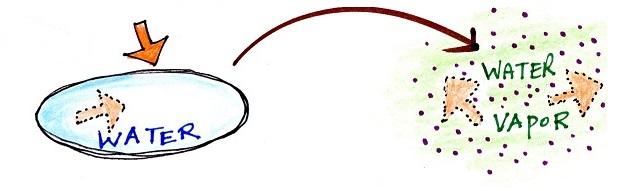
melting, liquid to gas is evaporation, and sublimation is
a solid to gas phase change.
Dry ice is the best example of sublimation that I can think of. When placed in a warm room, dry ice turns directly from solid carbon dioxide to gaseous carbon dioxide without melting first. If you wash clothes and stick them outside on a dry cold (below freezing) day they will eventually dry. The clothes would first freeze but then the ice would slowly sublime away.
In each case above energy must be added to the material changing phase. You can consciously add or supply the energy (such as when you put water in a pan and put the pan on a hot stove and cause it to boil).
That much is pretty clear. The confusing part of this topic is when phase changes occur without you playing any role. Energy is still required to melt ice; in this case the needed energy will be taken from the surroundings. It is not always obvious what the "surroundings" are. When you take energy from the surroundings, the surroundings will cool.
Dry ice is the best example of sublimation that I can think of. When placed in a warm room, dry ice turns directly from solid carbon dioxide to gaseous carbon dioxide without melting first. If you wash clothes and stick them outside on a dry cold (below freezing) day they will eventually dry. The clothes would first freeze but then the ice would slowly sublime away.
In each case above energy must be added to the material changing phase. You can consciously add or supply the energy (such as when you put water in a pan and put the pan on a hot stove and cause it to boil).
That much is pretty clear. The confusing part of this topic is when phase changes occur without you playing any role. Energy is still required to melt ice; in this case the needed energy will be taken from the surroundings. It is not always obvious what the "surroundings" are. When you take energy from the surroundings, the surroundings will cool.
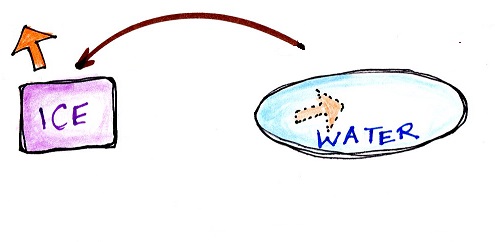
Here are a couple of examples
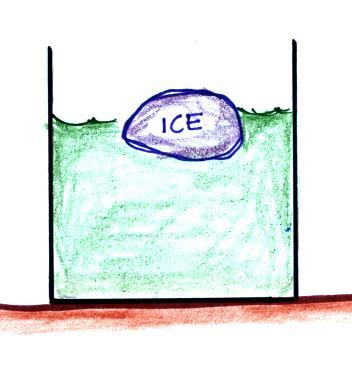
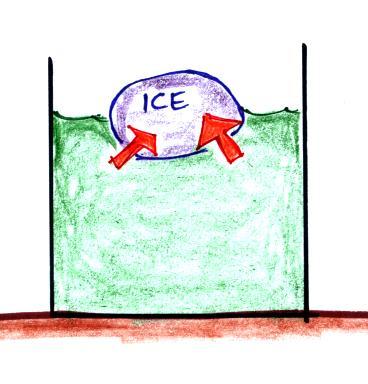
Energy will naturally flow from hot to cold; in this case from the water (about 70 F) to the ice (32 F). This transport of energy would occur via conduction.
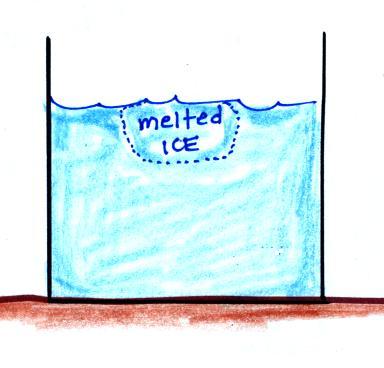
Energy is taken from the water and used to melt the ice. Because energy is taken from the water, the water cools.
Here's another, maybe even better, example because it's something you can experience and feel.
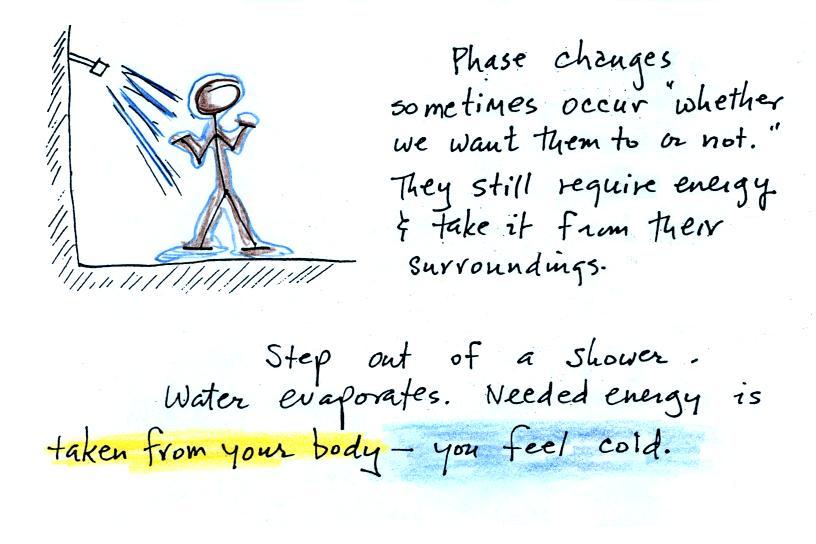
When you step out of the shower in the morning you're covered with water. Some of the water evaporates. It doesn't ask permission, it just evaporates whether you want it to or not. The energy needed for evaporation is taken from the surroundings, the surroundings in this case are your body. Because your body is losing energy you feel cold.
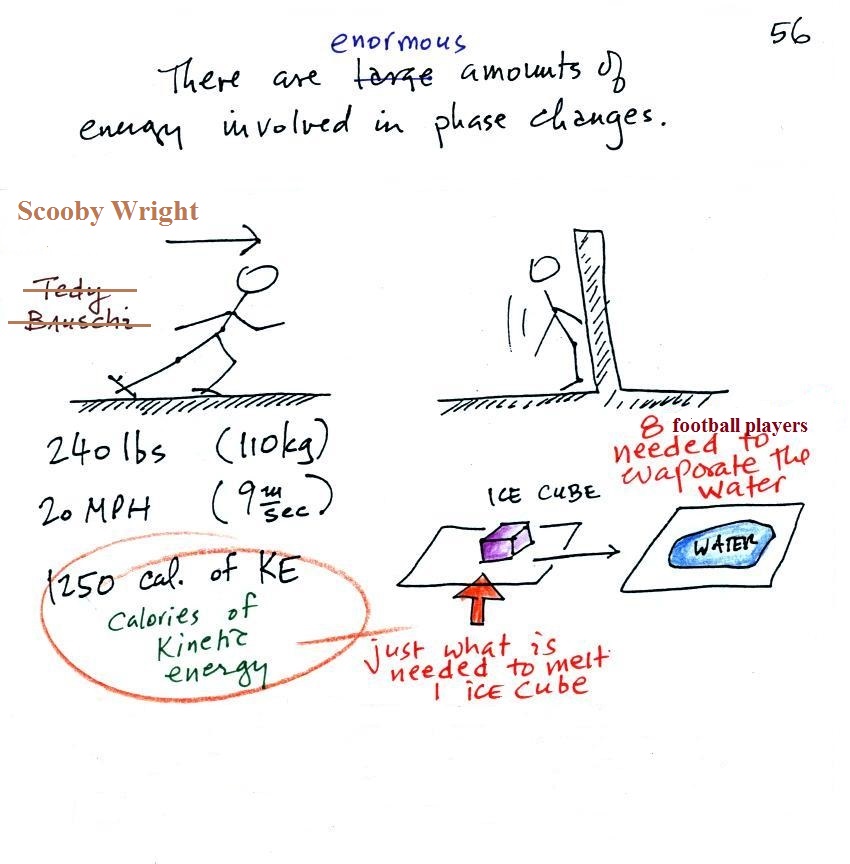
The object of this figure is to give you some appreciation for the amount of energy involved in phase changes. A 240 pound man or woman running at 20 MPH has just enough kinetic energy (if you could capture it) to be able to melt an ordinary ice cube (I have been using Tedy Bruschi as an example for several years but he's now retired so I have switched to Scooby Wright). It would take 8 people running at 20 MPH to evaporate the resulting ice water.
Latent heat energy is energy that is hidden in water or water vapor.
 |
 |
| Energy added to melt the ice is
hidden in the water that results |
Energy added to
evaporate the water is added to the energy already
in the water and is hidden in the water vapor |
 |
|
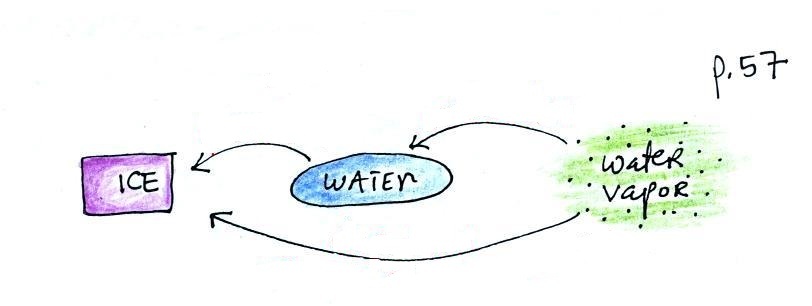
Again, try to name each phase change
and show the direction of energy flow (into or out of
the material) when the phase change occurs

You might not have heard of
deposition before when a gas changes directly to a
solid. The formation of frost is an example of
deposition.
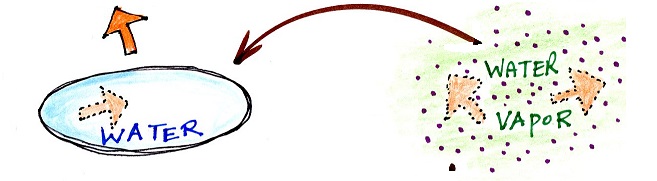
You can consciously remove energy from water vapor to make it condense. You take energy out of water to cause it to freeze (you could put water in a freezer; energy would flow from the relatively warm water to the colder surroundings). If one of these phase changes occurs, without you playing a role, energy will be released into the surroundings (causing the surroundings to warm).
Note the direction of the energy arrows - energy is being released into the surroundings (warming the surroundings). It's kind of like a genie coming out of a magic lamp. One Scooby Wright worth of kinetic energy is released when enough water freezes to make an ice cube. Many Scooby Wrights are released when water vapor condenses.
This release of energy into the surroundings and the warming of the surroundings is a little harder for us to appreciate because it never really happens to us in a way that we can feel. Have you ever stepped out of an air conditioned building into warm moist air outdoors and had your glasses or sunglasses "steam up"? Water vapor never condenses onto your body (your body is too warm). However if it did you would feel warm. It would be just the opposite of the cold feeling when you step out of the shower or a pool and the water on your body evaporates. You know how cold the evaporation can make you feel, the same amount of condensation would produce a lot of warming. I suspect we'd be surprised at how much warming it produces.
You can consciously remove energy from water vapor to make it condense. You take energy out of water to cause it to freeze (you could put water in a freezer; energy would flow from the relatively warm water to the colder surroundings). If one of these phase changes occurs, without you playing a role, energy will be released into the surroundings (causing the surroundings to warm).
Note the direction of the energy arrows - energy is being released into the surroundings (warming the surroundings). It's kind of like a genie coming out of a magic lamp. One Scooby Wright worth of kinetic energy is released when enough water freezes to make an ice cube. Many Scooby Wrights are released when water vapor condenses.
This release of energy into the surroundings and the warming of the surroundings is a little harder for us to appreciate because it never really happens to us in a way that we can feel. Have you ever stepped out of an air conditioned building into warm moist air outdoors and had your glasses or sunglasses "steam up"? Water vapor never condenses onto your body (your body is too warm). However if it did you would feel warm. It would be just the opposite of the cold feeling when you step out of the shower or a pool and the water on your body evaporates. You know how cold the evaporation can make you feel, the same amount of condensation would produce a lot of warming. I suspect we'd be surprised at how much warming it produces.
 |
 |
 |
|
Alternate view
showing the latent heat energy in water vapor
and water coming out of hiding during a phase
change and being released into the surroundings.
Here's a practical application of what we have been learning.
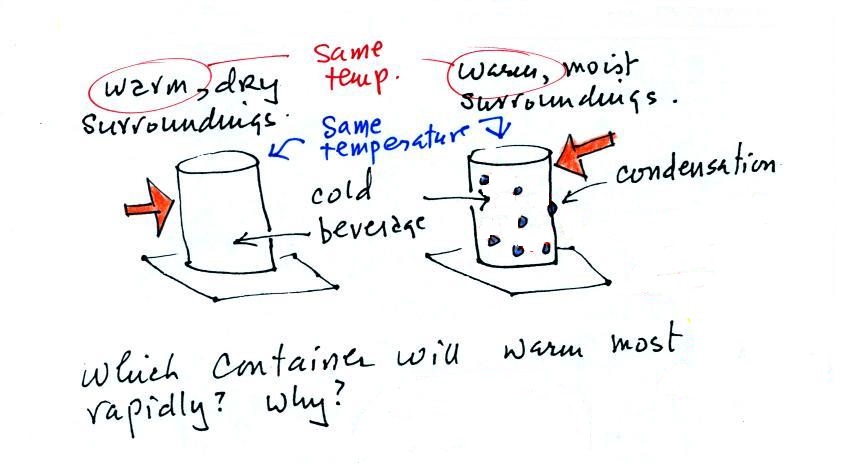
Cans of a cold drink are taken out of the refrigerator and placed on the kitchen table on a warm dry day and a warm humid day. Except for the differences in the amount of moisture in the air everything else is the same. Moisture has condensed onto the can above at right. Do the two cans warm up at the same rate or does one warm up more quickly than the other. In the latter case which can warms up most rapidly.
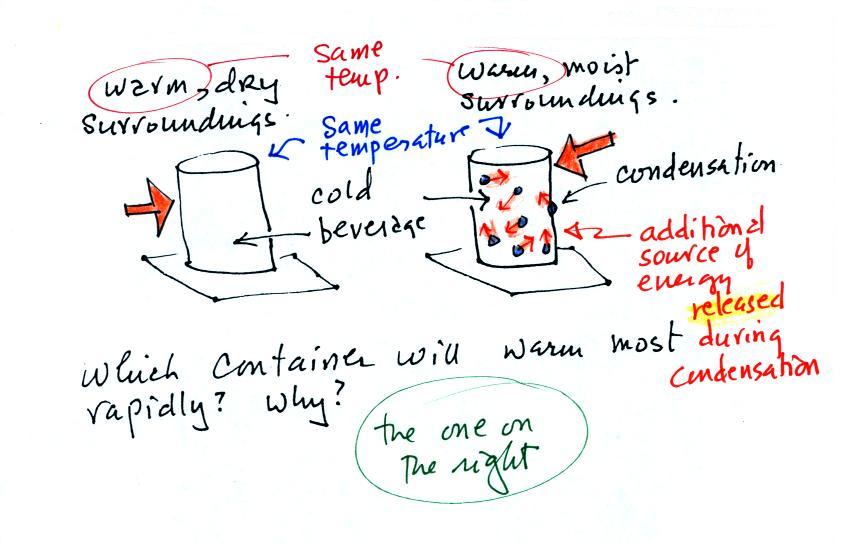
The can on the right will warm more quickly. Equal amounts of heat will flow from the warm air into the cold cans in both cases. Condensation of water vapor is an additional source of energy and will warm that can more rapidly. I suspect that the condensation may actually be the dominant process.

The foam "cozy", "koozie", or whatever you want to call it, that you can put around a can of soda or beer is designed to insulate the can from the warmer surroundings but also to keep water vapor in the air from condensing onto the can (source of the image above)
Here's a practical application of what we have been learning.

Cans of a cold drink are taken out of the refrigerator and placed on the kitchen table on a warm dry day and a warm humid day. Except for the differences in the amount of moisture in the air everything else is the same. Moisture has condensed onto the can above at right. Do the two cans warm up at the same rate or does one warm up more quickly than the other. In the latter case which can warms up most rapidly.

The can on the right will warm more quickly. Equal amounts of heat will flow from the warm air into the cold cans in both cases. Condensation of water vapor is an additional source of energy and will warm that can more rapidly. I suspect that the condensation may actually be the dominant process.

The foam "cozy", "koozie", or whatever you want to call it, that you can put around a can of soda or beer is designed to insulate the can from the warmer surroundings but also to keep water vapor in the air from condensing onto the can (source of the image above)
We're beating this
concept to death but we're almost done. Two
more figures to illustrate how latent heat energy
transport can carry energy from location to
another.
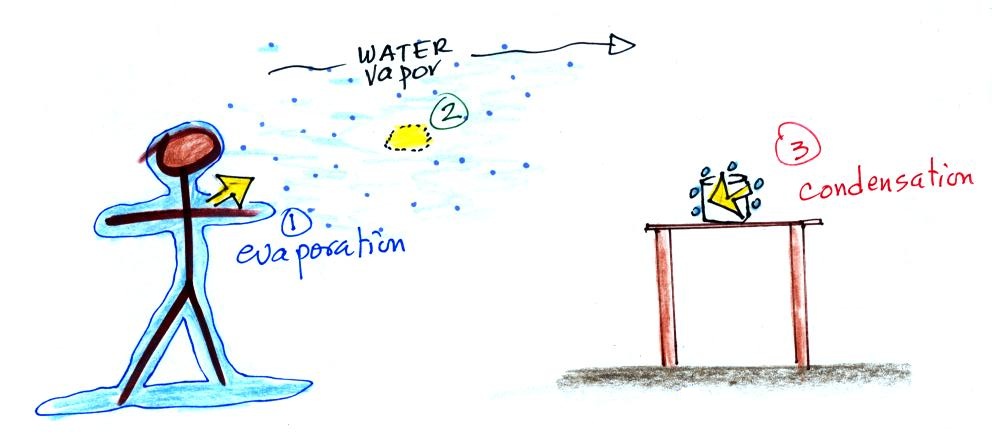
1. You've just stepped out of
the shower and are covered with water. The water
is evaporating and energy is being taken from your
body.
2. The water vapor (containing latent heat energy, the energy taken from your body), drifts into the kitchen where it finds a cold can sitting on a table.
3. Water vapor comes into contact with the cold can and condenses. The hidden latent heat energy in the water vapor is released into the can and warms the drink inside.
Without you even leaving the bathroom,
energy has effectively been transported from your warm body to the cold can in the kitchen.
Here's what happens on a much grander scale in the
atmosphere.2. The water vapor (containing latent heat energy, the energy taken from your body), drifts into the kitchen where it finds a cold can sitting on a table.
3. Water vapor comes into contact with the cold can and condenses. The hidden latent heat energy in the water vapor is released into the can and warms the drink inside.
Without you even leaving the bathroom,
energy has effectively been transported from your warm body to the cold can in the kitchen.
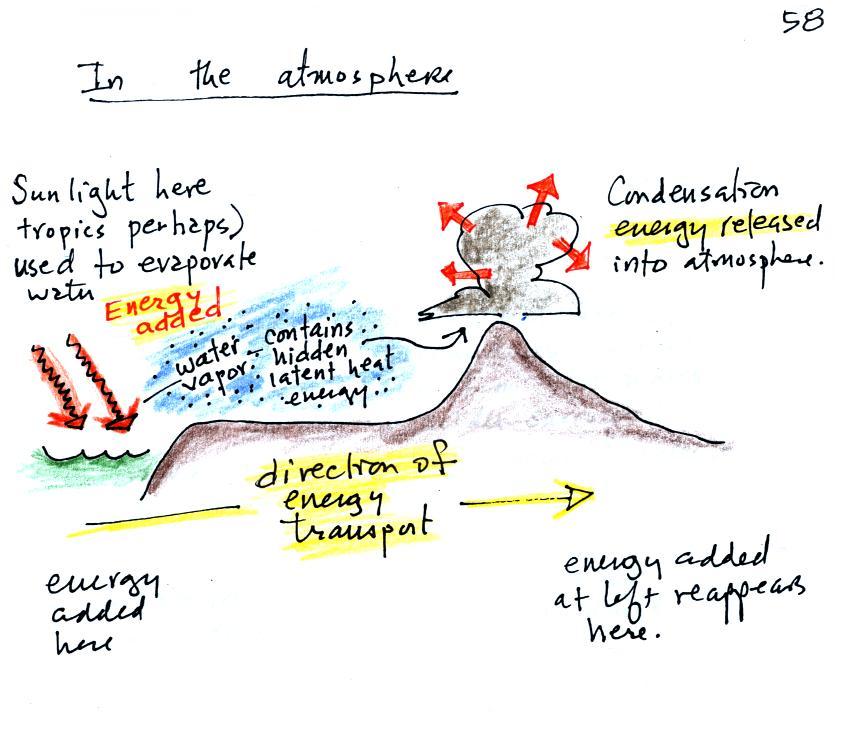
We start in this picture in the tropics
where there is often a surplus of sunlight energy.
Some of the incoming sunlight evaporates ocean
water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This
hidden energy reappears when something (air running into a
mountain and rising, expanding, and cooling) causes the
water vapor to condense. The condensation releases
energy into the surrounding atmosphere. This would
warm the air.
Energy arriving in sunlight in the tropics has effectively been transported to the atmosphere in a place like Tucson.
It's not clear how much time will be left in the period at this point, but I've included a little additional material nonetheless.
Energy arriving in sunlight in the tropics has effectively been transported to the atmosphere in a place like Tucson.
It's not clear how much time will be left in the period at this point, but I've included a little additional material nonetheless.
Energy transport by
electromagnetic radiation
It's time to tackle electromagnetic (EM) radiation, the 4th and most important of the energy transport processes.
It's time to tackle electromagnetic (EM) radiation, the 4th and most important of the energy transport processes.

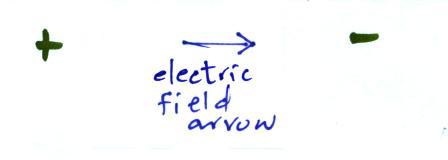
Many introductory textbooks depict EM
radiation with a wavy line like shown above. They
don't usually explain what the wavy line represents.
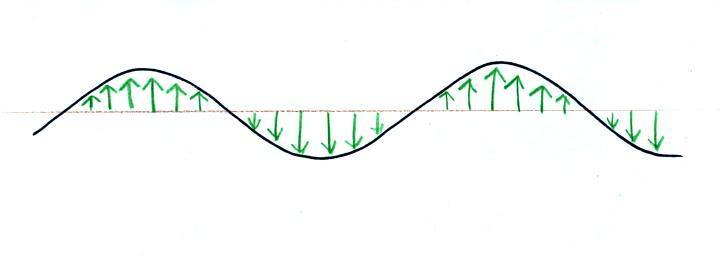
The wavy line just connects the tips
of a bunch of "electric field arrows". But what exactly
are electric field arrows?
Static electricity and electric fields
Static electricity and electric fields

To understand electric fields we need to first step back and review a couple of rules concerning static electricity.
That won't take too long, static electricity is something you're most likely already familiar with.
Believe it or not there is even a National Static Electricity Day (Jan. 9).
The static electricity rules are found at the top of p. 59 in the photocopied ClassNotes
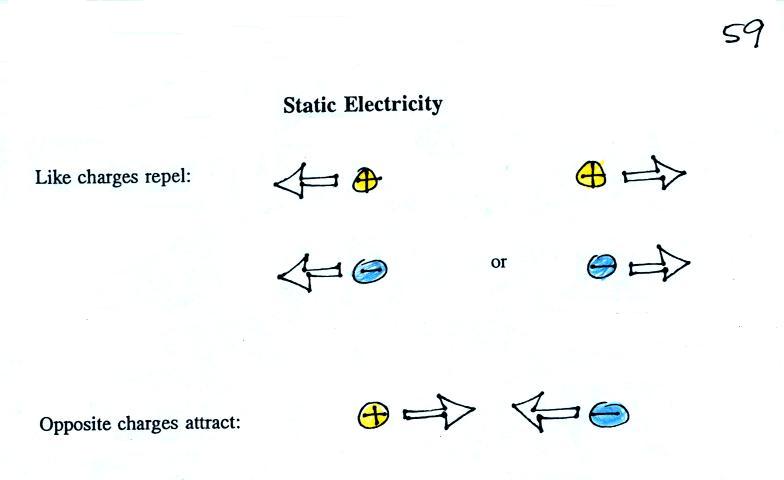
Two electrical charges with the same polarity (two positive charges or two negative charges) push each other apart. Opposite charges are attracted to each other. Here are some pictures I found online.
 |
 |
| This girl became charged with
static electricity while jumping on a trampoline
and illustrates the repulsive force of like
charges. Her hair and body are all
charged up with charge of the same polarity.
We don't know what polarity it is. The charge on her hair is trying to get as far away from charge on her body. People's hair will sometimes stand on end under a thunderstorm. That is a very dangerous situation to be in. This photo was a National Geographic Magazine 2013 Photo Contest winner (source) |
A cat covered in Styrofoam
"peanuts". Here the cat and the
"peanuts" have opposite charges and are
attracted to each other. Being a cat owner I would worry about the cat swallowing one of the peanuts and possibly choking. (source) |
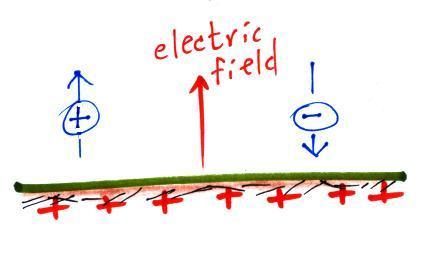
An electric
field arrow (vector)
shows the direction and strength
of the electrical force
exerted on a positive charge at that position.
shows the direction and strength
of the electrical force
exerted on a positive charge at that position.
Example questions
Here are a couple of questions to test your understanding.
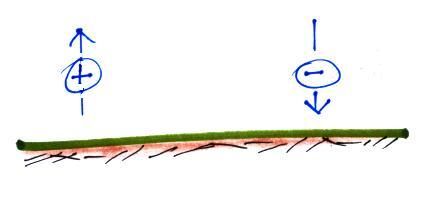
First what polarity of charge must be on ground to cause the charges in the figure below to move as they are doing. Would the electric field arrow in the air just above the ground point UPWARD, point DOWNWARD, or would the electric field arrow be ZERO?
Here's a second somewhat harder question

You'll find answers to both questions at the end of today's notes.