Wednesday Jan. 13, 2016
A couple of songs from Lissie:
"Record
Collector" (4:14) (recorded in the studios of KCMP FM,
Minnesota Public Radio), and "In Sleep"
(5:23) part of a set at the end of the Guitar
Center's Singer/Songwriter 2 Competition Finals at Hotel
Café in Hollywood (Mar., 2013) to check out the audio
system in ILC 150.
Several more songs from the same artist are included below.
"They All
Want You" (4:14), and "Further
Away" (4:14) from the Bing Lounge at 101.9 KINK FM
(Portland, OR).
"Love
in the City" (3:46), "The Habit"
Live at Hotel San Jose, SXSW 2013 (4:26)
Today's lecture notes are shown below.
Information about this class and course requirements
ATMO 170 is off and running once again. We
first briefly discussed the Course
Information handout. Please read through that information
carefully on your own and let me know if you have any
questions or concerns.
A textbook is not required for this class. If you want a
different and more complete picture of the subject, you might want
to purchase one of the textbooks that are being used in the other
ATMO 170A1 sections. Or if you'd like to borrow one of the instructor copies of introductory
level textbooks that I have in my office, just let me
know. Otherwise you should be able to do perfectly well in
the class by reading the online notes. And you should read
the online notes even if you are in class.
A set of photocopied ClassNotes (available in the ASUA
Bookstore in the Student Union) is "required." You should
try to purchase a copy as soon as you can because we may well be
using them in class on Friday. If you know someone with
notes leftover from the Fall or Spring 2015 semesters they will
work fine.
Writing is an important part of
this class and is described in more detail on the Writing Requirements handout.
Please have a careful look
at that also and
let me know if you have any questions.
The first half of your writing grade is an experiment
report. You only need to do one of the experiments, so think
about which of the experiments (listed on the handout) you might
like to do. I'll bring a signup sheet to class on
Friday. I'm also planning on bringing about 40 sets of
Experiment #1 materials to class on Friday for checkout.
Checkout is first come first served. Materials for the other
experiments will be handed out at roughly 3-week intervals.
The so-called One Side of One Page (1S1P) reports make up the
second part of your writing grade. Topics will appear
periodically during the semester on the class webpage. As
you write reports you will earn points (the exact number of points
will depend on the topic and the quality of your report).
Your goal should be to earn 45 1S1P pts, the maximum number
allowed, by the end of the semester.
You'll be allowed to revise and raise your grade on the first
draft of your experiment report. So you should be able to
earn a pretty high score on that. And, unless you
procrastinate, you can just keep on writing 1S1P reports until
you've earned 45 points. There's no reason not to earn a
high writing grade. The writing grade gets averaged in with
your quiz scores and, as the example below shows, can have a
significant and beneficial effect on your overall grade.
Grade example
Your final grade in this class will depend on your quiz scores,
how much extra credit you earn (from optional take home and in
class assignments), your writing grade, and (perhaps) your score
on the final exam. A sample grade report from one of the
Fall 2015 sections of this class is shown below (most of the
numbers are class averages).
Doe_J
quiz1 -42 (170 pts possible) 75.3% quiz scores
quiz2 -51 (175 pts possible) 72.9%
quiz3 -47 (175 pts possible) 73.1%
quiz4 -52 (175 pts possible) 70.3%
2.5 EC points (3.3 pts possible) extra credit earned on optional assignments
writing scores
writing scores: 32.0 (expt/book report) + 45.0 (1S1P pts)
writing grade: 96.3%
overall averages (prior to the Final
Exam)
average (no quiz scores dropped): 77.6% + 2.5 =
80.0%
average (lowest quiz score dropped): 79.4% + 2.5 = 81.9%
Final exam score: 76.0%
Overall grade:
80.7% (B)
The 4 quiz grades are shown at the top.
Students that did turn in the Optional Assignments
earned on average 2.5 pts of extra credit during the
semester. You will have the opportunity to earn at least 3
extra credit points.
A score of 32 points (out of 40) on the experiment report and 45
1S1P points resulted in a writing percentage grade of 96.3%.
There's no good reason not to end up with a writing score close to
100% (or even greater than 100%)
The overall average without any quiz scores dropped is shown
next. Since the result, 80.0%, is less than 90.0% the
average student last fall did have to take the final exam
The second average (with the lowest score dropped) is a little
higher, 81.9%.
If you do well on the final exam it will count 40% of
your overall grade (trying to maximize the benefit it can
have). If you don't do so well on the final it only counts
20% (minimizing the damage it can cause). In this example
the final exam score (76%) was lower than the 81.9% value, so the
final exam only counted 20% and the overall score was 80.7%.
Be sure to note that even with C grades on each of the quizzes and
a C on the Final Exam you could well end up with a B in the
class. That is possible when you have a high writing grade
and also have some extra credit points.
"Chapter 1" - the earth's atmosphere
We did cover a little course material on this
first day of class so that you can get an idea of how that will
work. Also I won't feel so bad about not covering new
material on the last day of class in May.
If we were using a book
we'd start in Chapter 1 and here's some of what we would first
be looking at in this course.
We will come back to the first item, the
composition of the atmosphere, today.
Before we do that however, here
are a few questions to get you thinking about the air around
you. We didn't really cover any
of this in class.
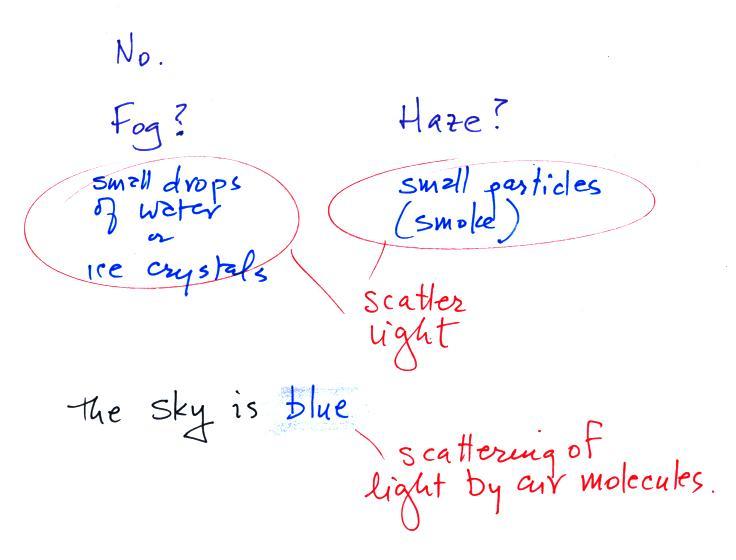
Can we see air?
Air is mostly clear, transparent, and invisible. Most
gases are invisible. Sometimes the air looks foggy,
hazy, or smoggy. In those cases you are probably
"seeing" small water droplets or ice crystals (fog) or small
particles of dust or smoke (haze and smog). The
particles themselves may be too small to be seen with the
naked eye but are visible because they scatter (redirect)
light. I didn't really mention or explain what
that is but it's a pretty important concept and we
will learn more about it soon.
And to be completely honest air isn't really invisible.
If you shine a bright light through enough air, such as when
sunlight shines through the atmosphere, the air (the sky)
appears blue. This is a little more complicated form of
scattering of sunlight by air molecules. We'll come back
to this later as well.
Can you
smell air?
I don't think you can smell or taste air (air containing
nitrogen, oxygen, water vapor, argon and carbon
dioxide). But there are also lots of other odors you can
sometimes smell (freshly cut grass, hamburgers on a grill,
etc). I don't consider these normal constituents of
the atmosphere.
You can probably also smell certain pollutants. I
suspect our sense of smell is sensitive enough for us to
detect certain air pollutants even when their concentration
is very small (probably a good thing because many of them
are poisonous).
Natural gas (methane) used in hot water
heaters, some stoves, and furnaces is odorless. A
chemical (mercaptan) is added to natural gas so that you can
smell it and know when there is a leak before it builds up
to a concentration that could cause an explosion.
It is harder to answer this question.
We're always in contact with air. Maybe we've grown so
accustomed to it we aren't aware of how it feels. We can
certainly feel whether the air is hot or cold, but that have
more to do with energy exchange between us and our
surroundings. And we can feel wind.
In a couple of weeks we will see that, here in the classroom,
air pressure is pressing on every square inch of our bodies with
12 or 13 pounds of force. If that were to change suddenly
I'm pretty sure we'd feel it and it would probably really hurt.
2 objectives for today:
1. You should be able to list the 5 most abundant gases in air
and say something (maybe more than one thing) about
each of them
2. You should be able to define or explain
dew point temperature
and you should know the meaning of the term monsoon.
Let's start with the most abundant gas in the atmosphere. I
poured some of that material (in liquid form) into a Styrofoam
cup. Here's a photo I took back in my office.
You can see the liquid, it's
clear, it looks like water. Probably a lot of you
knew this was nitrogen. Liquid nitrogen is very cold
and begins to boil (evaporate) at -321o
F.
The most abundant gas in the earth's atmosphere is
nitrogen. We'll use liquid nitrogen in several class
demonstration this semester mostly because it is so
cold.
Nitrogen was discovered in 1772 by Daniel Rutherford (a
Scottish botanist). Atmospheric nitrogen is relatively
unreactive and is sometimes used to replace air in packaged
foods to preserve freshness. You don't need to worry
about details like this for a quiz.
Oxygen is the second most abundant gas in the
atmosphere. Oxygen is the most abundant element (by
mass) in the earth's crust, in ocean water, and in the human
body. In liquid form it also becomes visible.
Some photographs of liquid oxygen (O2)
are shown above
(it
boils at -297o F).
It has a (very faint) pale blue color (I
was pretty disappointed when I first saw it because I had
heard it was blue and imagined it was a deeper more vivid
blue). When
heated (such as in an automobile engine) the oxygen
and nitrogen in air react to form compounds such as
nitric oxide (NO), nitrogen dioxide (NO2),
and nitrous oxide (N2O). Together as
a group these are called oxides of nitrogen; the first
two are air pollutants, the last is a greenhouse
gas. I'd
love to bring some liquid oxygen to class but
I'm not sure it's available on campus and you
probably need to be careful with it because it
is reactive.
I recently learned that liquid ozone (O3)
does have a nice deep blue color.
Liquid ozone (source
of this photograph).
It's probably very hard to find and is
also very (dangerously) reactive. Ozone gas is also
poisonous.
Here is a complete
list of the 5 most abundant gases in air. And a note about the figures you'll find in these
online notes. They may differ somewhat from
those drawn in class. I often redraw them after class, or
use neater versions from a previous semester for improved clarity
(and so I can get the notes online more quickly).
With a little practice you should be able to start
with a blank sheet of paper and reproduce the list below.
Water vapor and argon are the 3rd and 4th most abundant
gases in the atmosphere. A 2% water vapor concentration is
listed above but it can vary from near 0% to as high as 3% or
4%. Water vapor is, in many locations, the 3rd most abundant
gas in air. In Tucson most of the year, the air is dry
enough that argon is in 3rd position and water vapor is 4th.
Water vapor and carbon dioxide are circled because they are
greenhouse gases.
Water vapor, a gas, is invisible. Water is the only
compound that exists naturally in solid, liquid, and gaseous
phases in the atmosphere.
Argon is an unreactive noble gas (helium, neon, krypton, xenon, and radon are also inert gases).

Here's a
picture of solid argon ("argon ice"). It melts
at melts
at -309o F and
boils at -302o F;
it's doing both in this picture. (image source).
Here's a little more
explanation (from Wikipedia)
of why noble gases are so unreactive. You can gloss over
all these additional details if you want to, none of this was covered in class.
The noble gases have full valence electron shells. Valence electrons are the outermost electrons of an atom and are
normally the only electrons that participate in chemical bonding. Atoms
with full valence electron shells are extremely stable and
therefore do not tend to form chemical bonds and have little
tendency to gain or lose electrons (take electrons from or
give electrons to atoms of different materials).
Noble gases are often used used in "neon signs"; argon
produces a blue color. The colors produced by Argon (Ar),
Helium (He), Kryton (Kr), Neon (Ne) and Xenon (Xe), which are
also noble gases, are shown above (source
of the images). An electric current is
traveling through and heating the gas in the tube causing it to
emit light. You're seeing the light emitted by the gas
itself. The inert gases don't react with the metal
electrodes in the bulbs.
Fluorescent bulbs (including energy saving CFLs) often also
contain mercury vapor (which means you should properly dispose
of them when they burn out). The mercury vapor in CFL
bulbs emits ultraviolet light that strikes a phosphor coating on
the inside of the bulb. Different colors are emitted
depending on the particular type of phosphor used in the bulb.

This is solid carbon dioxide, better known as
dry ice. It doesn't melt, it sublimes. Sublimation
is a solid to gas phase change, evaporation is a liquid to gas
change. (
source of the
image above).
The concentration of carbon dioxide in air is much smaller
than the other gases (it's about 0.04% but you don't need to
remember the actual value). That doesn't mean it isn't
important. We'll spend a lot of time this semester talking
about water vapor and also carbon dioxide. Water vapor and
carbon dioxide are the two best known and most important
greenhouse gases. The greenhouse effect warms the
earth. Concentrations of greenhouse gases such as carbon
dioxide are increasing and there is concern this will strengthen
the greenhouse effect and cause global warming. That's a
topic we'll look at during the semester.
If we were using a textbook we'd probably find something like
the following table near the beginning of the book ( I found this
table a few years ago in a Wikipedia
article about the earth's atmosphere ).
I like our list of the 5 most abundant gases better. It's
much more manageable. There is almost too much information
in a chart like this, you might be overwhelmed and not remember
much. Also unless you are familiar with the units on the
numbers they might be confusing. And notice you don't find
water vapor in 3rd or 4th position near the top of the
chart. That's because this is a list of the gases in dry
air. Unless you're very attentive, you might miss that fact
and might not see water vapor way which is included at the bottom
of the chart.
If you click on the link above to the Wikipedia article on the
earth's atmosphere, you'll find that the list above has been
replaced with a shorter simpler list (much more like the one we
created in class).
Dew point temperature and the
summer monsoon
Water plays many important roles in the
atmosphere. One of them is the formation of clouds, storms,
and precipitation. Meteorologists are very interested in
knowing and keeping track of how much water vapor is in the
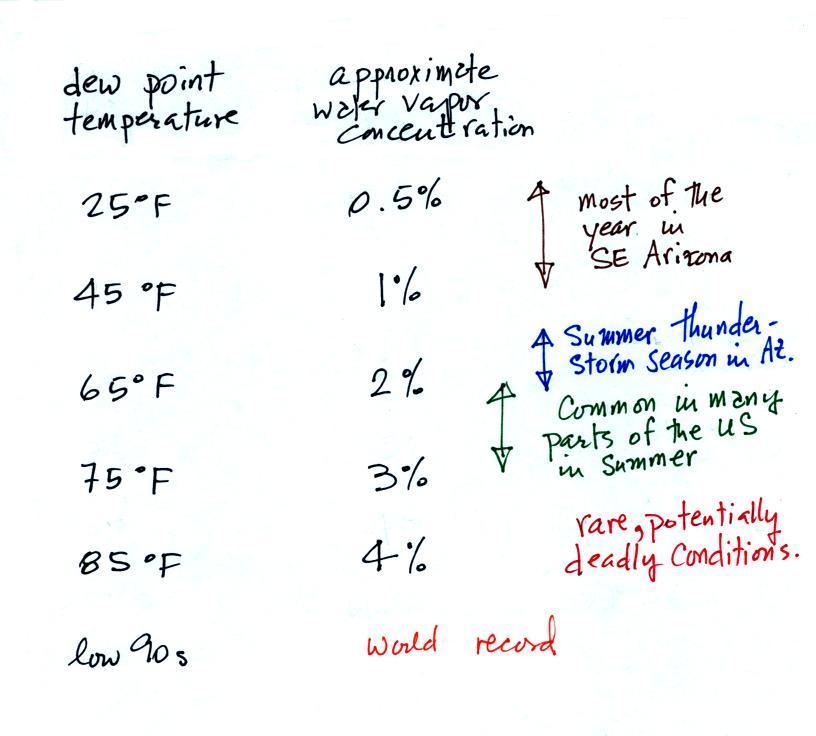
air. One of the variables they use is the dew point
temperature. The value of the dew point gives you an idea of
how much water vapor is actually in the air. A high dew
point value means a higher the water vapor concentration.

The chart below gives a rough equivalence between
dew point temperature and percentage concentration of water
vapor in the air.
Note that for every 20 F increase in dew
point temperature, the amount of water vapor in the air
roughly doubles.
Air temperature will always be equal to or warmer than the
dew point temperature. Experiencing 80o F dew points would be
very unpleasant and possibly life threatening because your
body might not be able to cool itself ( the air
temperature would probably be in the 90s or maybe even
warmer). You could get
heatstroke and die.
Click here
to see current dew point temperatures across the U.S. Here's
a
link concerning unusually high, even record setting dew
point temperatures.
This is as far as we got
today in class. I've moved material on the summer
monsoon and the dew point's "2nd job" to the start of the
Fri., Jan. 15 classnotes.