Wednesday, March 23, 2016
It seemed like a good time for something from the Buena Vista
Social Club given President Obama's trip to Cuba earlier this
week. Songs on the first line below are from the original
group I believe. The performance at the White House featured
some original and newer members of the group.
Buena Vista Social Club "La Negra
Tomasa" (8:29), "El Cuarto de
Tula" (8:28), "Chan Chan"
(live at Carnegie Hall) (3:22),
Buena Vista
Social Club Orquesta at the White House (Oct. 2015).
A take home Optional
Assignment was handed out in class today. It is
due next Wednesday (Mar. 30) [you should have the assignment
done before coming to class on the due date].
I'll have copies of the assignment with me in class on Friday and
Monday next week.
We'll work through a few of the following humidity
variable example problems in class today. The goal
here is for you to become a little more familiar with the
humidity variables and see what causes them to change value.
Humidity example problem #1
There are 4 humidity variables (mixing
ratio, saturation mixing ratio, relative humidity, and
dew point temperature). Generally I'll give you
values for two of them and you'll need to figure out
values for the other two.
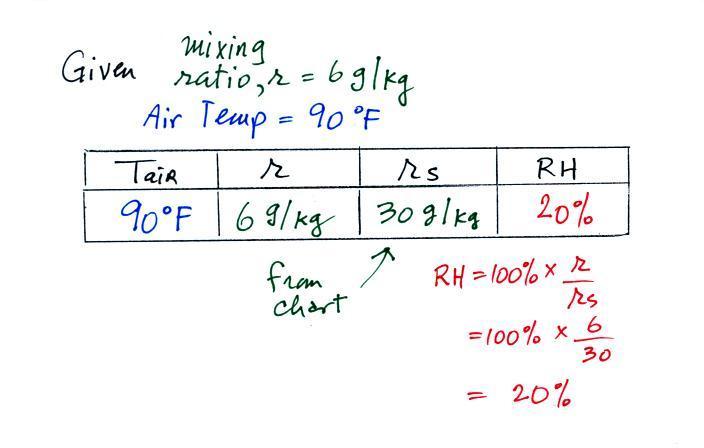
Here are the starting conditions for this first problem
Tair = 90 F
|
r = 6 g/kg
|
RH = ?
|
Td = ?
|
We start by entering the data we were given
Anytime you know the air's temperature you
can look up the saturation mixing ratio value on a chart (such
as the one on p. 86 in the ClassNotes); the saturation mixing
ratio is 30 g/kg for 90 F air. 90 F air could potentially
hold 30 grams of water vapor per kilogram of dry air (it
actually contains 6 grams per kilogram in this example).
Once you know mixing ratio and saturation mixing ratio you
can calculate the relative humidity (you divide the mixing ratio
by the saturation mixing ratio, 6/30, and multiply the result by
100%). You ought to be able to work out the ratio 6/30 in
your head (6/30 = 1/5 = 0.2). The RH is 20%.
The numbers we just figured out are shown on the top line
below.
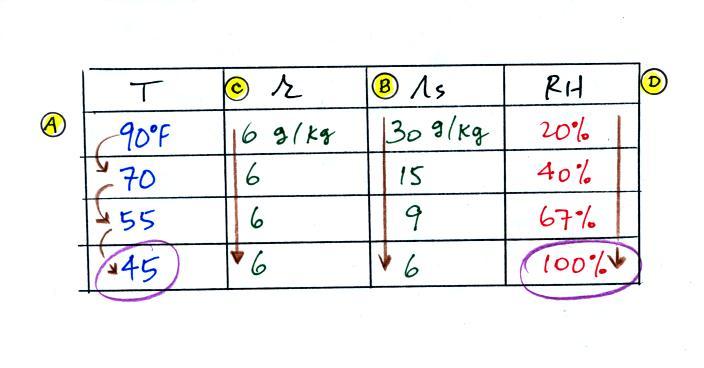
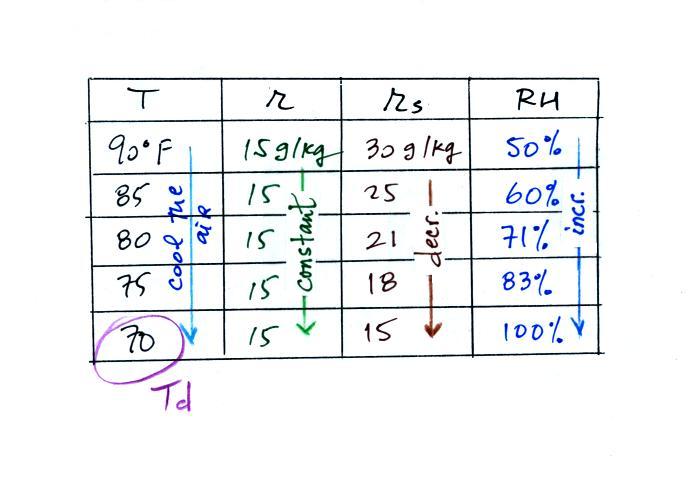
(A) To figure out the dew point, we imagine cooling
the air from 90F to 70F, then to 55F, and finally to
45F. Note the effect this has on the mixing ratio, the
saturation mixing ratio and the relative humidity.
(B) At each step we looked up the saturation mixing ratio
and entered it on the chart. Note that the saturation
mixing ratio values decrease
as the air is cooling.
(C) The mixing
ratio (r) doesn't change as we cool the air. The
only thing that changes r is adding or removing water vapor and
we aren't doing either. This is probably the most
difficult concept to grasp.
(D) Note how the relative humidity is increasing as we cool
the air. The air still contains the same amount of water
vapor it is just that the air's capacity is decreasing.
Finally at 45 F the RH becomes 100%. This is the dew
point. The dew point temperature is 45 F
What would happen if we cooled the air below the dew
point temperature?
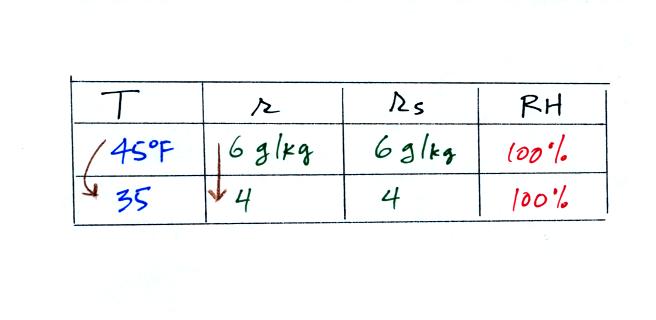
35 F air can't hold the 6 grams of water
vapor that 45 F air can. You can only "fit" 4 grams of
water vapor into the 35 F air. The remaining 2 grams would
condense. If this happened at ground level the ground
would get wet with dew. If it happens above the ground,
the water vapor condenses onto small particles in the air and
forms fog or a cloud. Because water vapor is being taken
out of the air (the water vapor is turning into water), the
mixing ratio will decrease from 6 g/kg to 4 g/kg. As you
cool air below the dew point, the RH stays constant at 100% and
the mixing ratio decreases.
In many ways cooling moist air is liking squeezing a
moist sponge
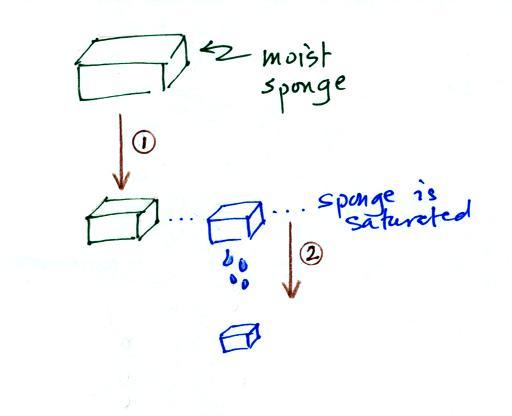
Squeezing the sponge and reducing its volume
is like cooling moist air and reducing the saturation mixing
ratio. At first (Path 1 in the figure) when you squeeze
the sponge nothing happens, no water drips out. Eventually
you get to a point where the sponge is saturated. This is
like reaching the dew point. If you squeeze the sponge any
further (Path 2) water will begin to drip out of the sponge
(water vapor will condense from the air).
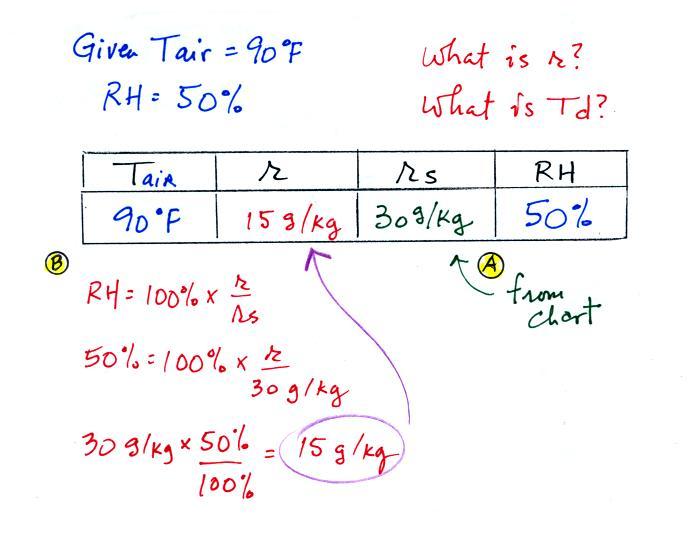
Humidity example problem #2
Tair = 90 F
|
r = ?
|
| RH = 50% |
Td = ?
|
The problem is worked out in detail below
First you fill in the air temperature and the RH data that
you are given.
(A) since you know the air's temperature you can look up the
saturation mixing ratio (30 g/kg).
(B) Then you might be able to figure out the mixing
ratio in your head. Air that could hold up to 30 g/kg of
water vapor is filled to 50% of its capacity. Half of 30
is 15, that is the mixing ratio. Or you can substitute
into the relative humidity formula and solve for the mixing
ratio. The details of that calculation are shown above
at B.
Finally you imagine cooling the air (I
added more intermediate temperatures in the table above
than we did in class). Notice how the
saturation mixing ratio decreases, the mixing ratio stays
constant, and the relative humidity increases as the air is
cooled. In this example the RH reached 100% when
the air had cooled to 70 F. That is the dew point
temperature.
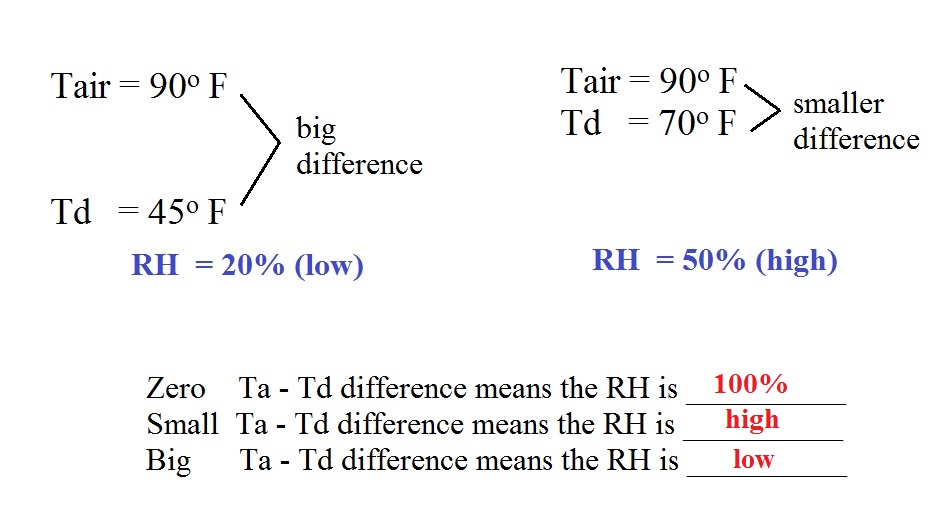
What does
the difference Tair - Td tell you about the
relative humidity?
We
can use results from humidity problems #1 and
#2 to learn and understand a useful
rule.
In the first example the difference between the air and dew
point temperatures was large (45 F) and the RH was low (20%).
In the 2nd problem the difference between the air and dew
point temperatures was smaller (20 F) and the RH was higher
(50%).
The easiest way to remember this rule might be to remember the
case where there is no difference between the air and dew
point temperatures.
The RH then would be 100%.
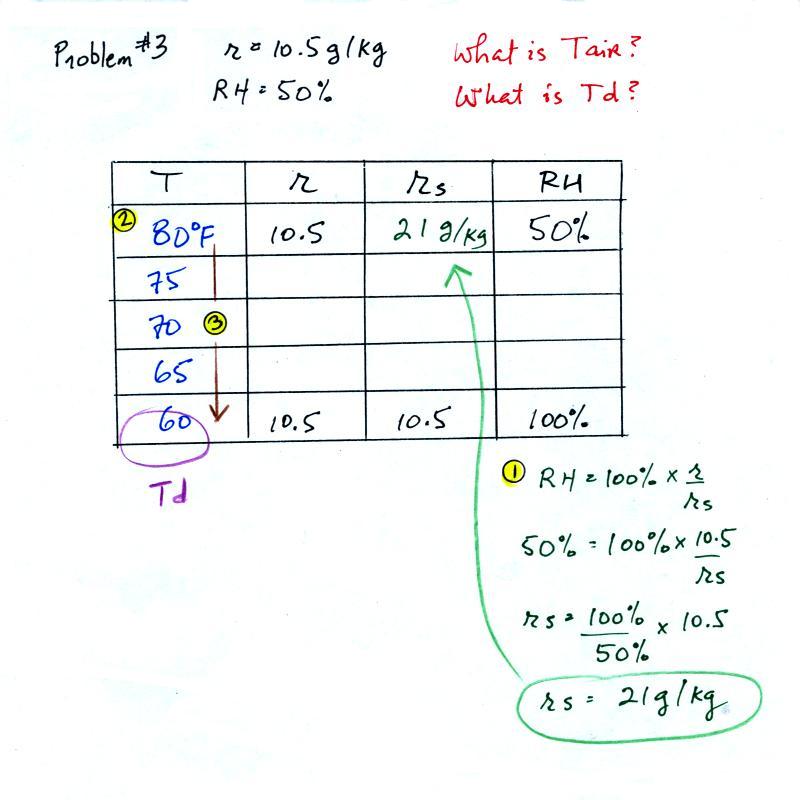
Humidity example problem #3
Tair =
?
|
r = 10.5 g/kg
|
| RH = 50% |
Td = ?
|
We skipped this problem in
class. But I've included all the details
below just in case you want to try to solve the problem on
your own.
You're given the the mixing ratio = 10.5 g/kg and a
relative humidity of 50%. You
need to figure out the air temperature and the dew point
temperature. Here's the play by play solution
to the question:
(1) The air contains 10.5 g/kg of water vapor. This is
50% (half) of what the air could potentially hold. So
the air's capacity, the saturation mixing ratio must be 21
g/kg (you can either do this in your head or use the RH
equation following the steps shown above).
(2) Once you know the saturation mixing ratio you can look up
the air temperature in a table (80 F air has a saturation
mixing ratio of 21 g/kg)
(3) Then you imagine cooling the air until the RH becomes
100%. This occurs at 60 F. The dew point is 60 F
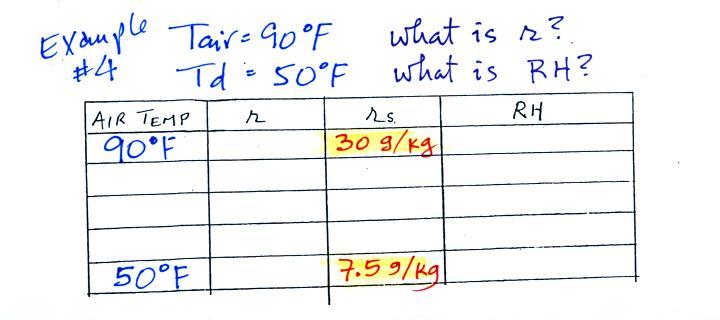
Humidity example problem #4
Tair = 90 F
|
r = ?
|
RH = ?
|
Td = 50 F
|
One of the dew
point's jobs is the same as the mixing ratio - it
gives you an idea of the actual amount of water vapor
in the air. This problem will show that if you
know the dew point, you can quickly figure out the
mixing ratio and vice versa. Knowing the dew
point is equivalent to knowing the mixing ratio.
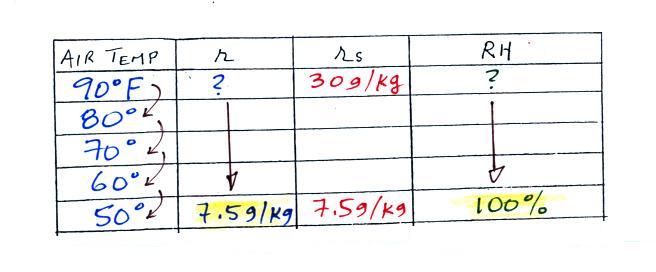
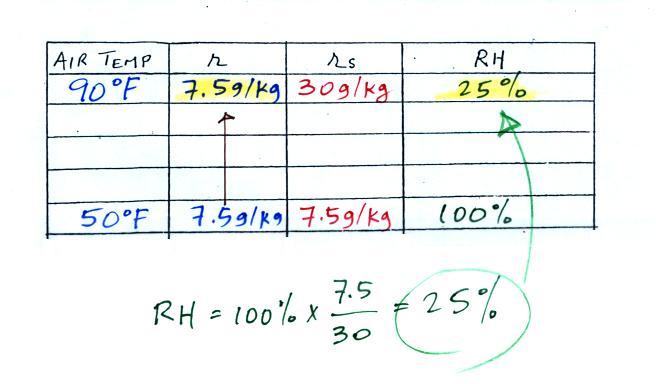
We enter the two temperatures given on a chart and look up the
saturation mixing ratio for each.
We ignore the fact that we don't know the mixing ratio.
We do know that if we cool the 90 F air to 50 F the RH will
become 100%. So on the 50 F row, we can set the mixing
ratio equal to the value of the saturation mixing ratio at 50
F, 7.5 g/kg. The two have to be equal in order for the
RH to be 100%.
Remember back to the three earlier examples.
When we cooled air to the the dew point, the mixing ratio
didn't change. So the mixing ratio must have been 7.5
all along. Once we know the mixing ratio in the 90
F air it is a simple matter to calculate the relative
humidity, 25%.
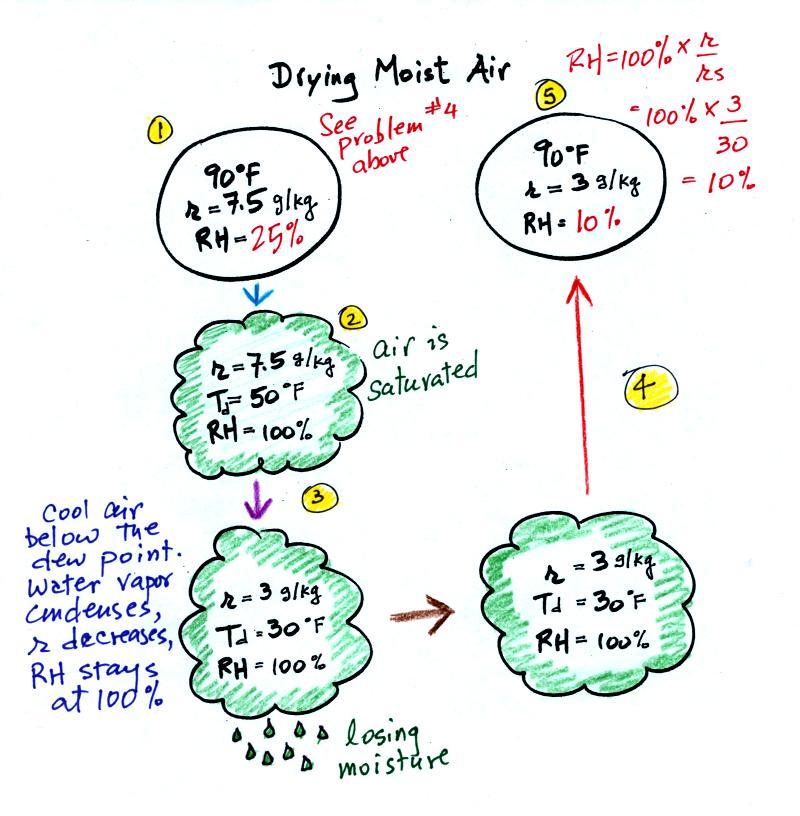
Drying moist air
The figure below is on p. 87 in the photocopied ClassNotes.
It
explains how you can dry moist air.
At Point 1 we start with some 90 F air with a relative
humidity of 25%, fairly dry air. These are the same
numbers that we had in Example Problem #4. We imagine
cooling this air to the dew point temperature, 50 F.
While doing that the mixing ratio, r, would stay
constant. Relative humidity would increase and
eventually reach 100%. A cloud would form (Pt. 2 in the
figure above).
Then we continue to cool the air below the dew point, to 30
F. Air that is cooled below the dew point finds itself
with more water vapor than it can contain. The excess
moisture must condense (we will assume it falls out of the air
as rain or snow). Mixing ratio will decrease, the
relative humidity will remain 100%. When air reaches 30
F it contains 3 g/kg, less than half the moisture that it
originally did (7.5 g/kg).
The air is being warmed back up to 90 F along Path 4. As
it warms the mixing ratio remains constant. Cooling
moist air raises the RH. Warming moist air, as is being
down here, lowers the RH. Once back at the starting
temperature, Point 5, the air now has a RH of only 10%.
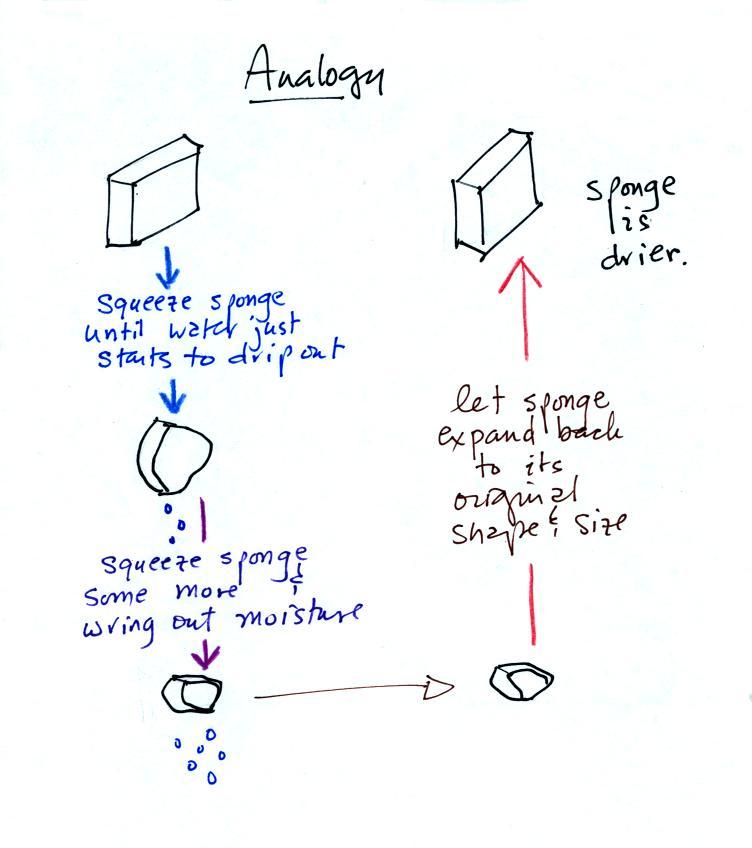
Drying moist air is basically wringing moisture from a wet
sponge.
You start to squeeze the sponge and it gets
smaller. That's like cooling the air and reducing the
saturation mixing ratio, the air's capacity for water
vapor. At first squeezing the sponge doesn't cause
anything to happen (that's like cooling the air, the mixing
ratio stays constant as long as the air doesn't lose any water
vapor). Eventually water will start to drop from the
sponge (with air this is what happens when you reach the dew
point and continue to cool the air below the dew point).
Then you let go of the sponge and let it expand back to its
original shape and size (the air warms back to its original
temperature). The sponge (and the air) will be drier
than when you started.
Dry air indoors in the winter
The air indoors in the winter is often quite
dry.
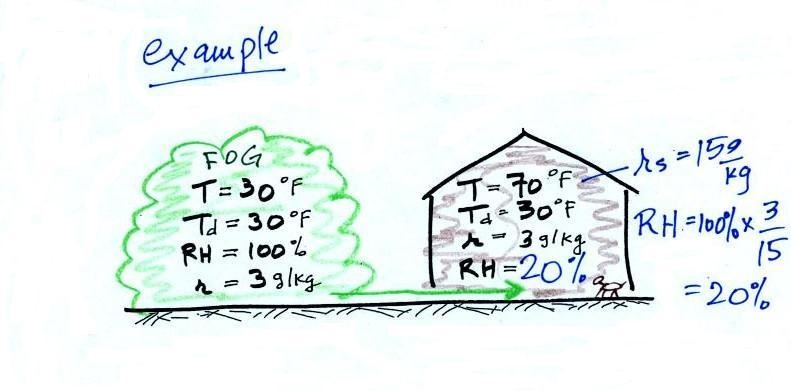
In the winter, cold air is brought inside your
house or apartment and warmed. Imagine foggy 30 F air
(with a RH of 100% this is a best case scenario, the cold air
outdoors usually has a relative humidity less than 100% and is
drier). Bringing the air inside and warming it will cause the
RH to drop from 100% to 20%.. This can cause chapped
skin, can irritate nasal passages, and causes cat's fur to
become charged with static electricity.
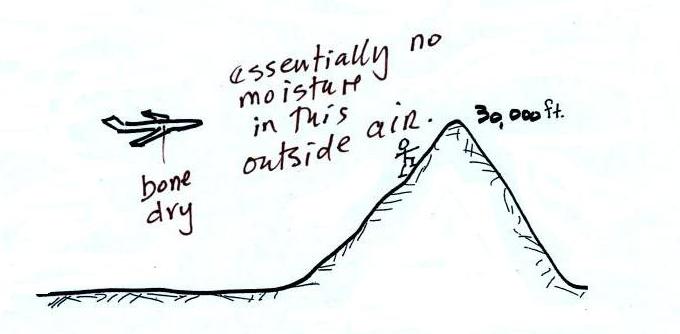
The air in an airplane comes from
outside the plane. The air outside the plane can
be very cold (-60 F perhaps) and contains very little
water vapor (even if the -60 F air is saturated it
would contain essentially no water vapor). When
brought inside and warmed to a comfortable
temperature, the RH of the air in the plane would be
essentially 0%. The RH doesn't get this low
because the airplane adds moisture to the air to make
to make the cabin environment tolerable. Still
the RH of the air inside the plane is pretty low and
passengers often complain of dehydration
on long airplane flights. This
may increase the risk of catching a cold (ref)
The rain-shadow effect
Next a much more important example of drying moist
air (see p. 88 in the photocopied ClassNotes).
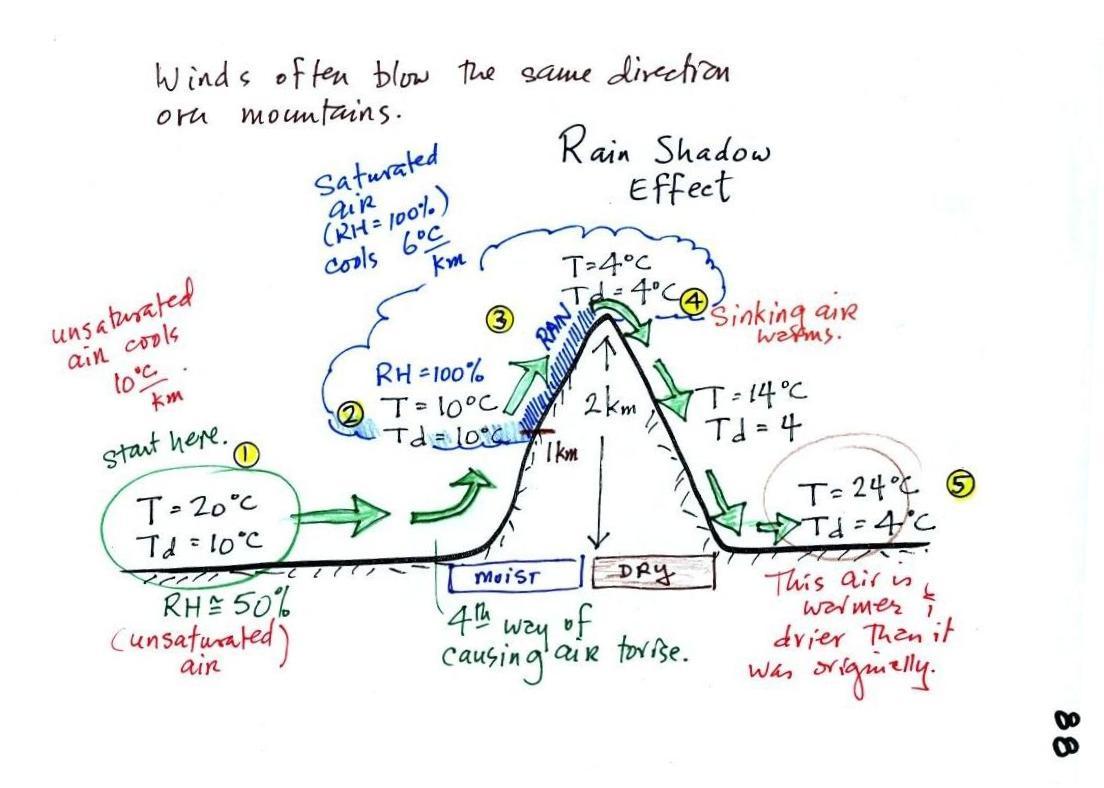
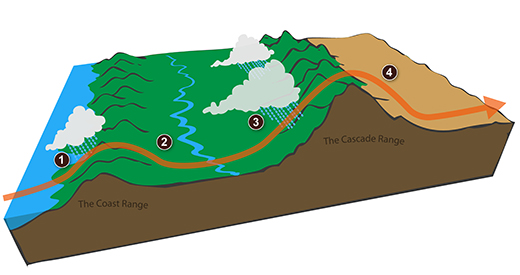
We start with some moist but unsaturated air (the
RH is about 50%) at Point 1 (the air and dew point
temperatures would need to be equal in order for the air
to be saturated).
As it is moving toward the right the air runs into a
mountain and starts to rise*
(see below).
Rising air expands and cools. Unsaturated air cools
10 C for every kilometer of altitude gain (this is known
as the dry adiabatic lapse rate but isn't something you
need to remember). So after rising 1 km the air will
cool to 10 C which is the dew point.
The air becomes saturated at Point 2 (the air temperature
and the dew point are both 10 C). Would you be able
to tell if you were outdoors looking at the
mountain? Yes, you would see a cloud appear.
Now that the RH = 100%, the saturated air cools at a
slower rate than unsaturated air (condensation of water
vapor releases latent heat energy inside the rising volume
of air, this warming partly offsets the cooling caused by
expansion). We'll use a value of 6 C/km (an average
value). The air cools from 10 C to 4 C in next
kilometer up to the top of the mountain. Because the
air is being cooled below its dew point at Point 3, some
of the water vapor will condense and fall to the ground as
rain. Moisture is being removed from the air and the
value of the mixing ratio (and the dew point temperature)
decreases.
At Point 4 the air starts back down the right side of the
mountain. Sinking air is compressed and warms.
As soon as the air starts to sink and warm, the relative
humidity drops below 100% and the cloud disappears.
The sinking unsaturated air will warm at the 10 C/km
rate.
At Point 5 the air ends up warmer (24 C vs 20 C) and drier
(Td = 4 C vs Td = 10 C) than when it started out.
The downwind side of the mountain is referred to as a
"rain shadow" because rain is less likely there than on
the upwind side of the mountain. Rain is less likely
because the air is sinking and because the air on the
downwind side is drier than it was on the upslope side.
This is as far as we got in
class today. I'll probably briefly mention the
two rain shadow effect examples below at the beginning
of class on Friday
*This
is topographic lifting, the 4th of 4 processes that can
cause air to rise. The other three were: convergence
(surface winds spiraling inward toward a low pressure
center will rise), fronts (both warm and cold fronts cause
air to rise), and convection (warm air rises).
|
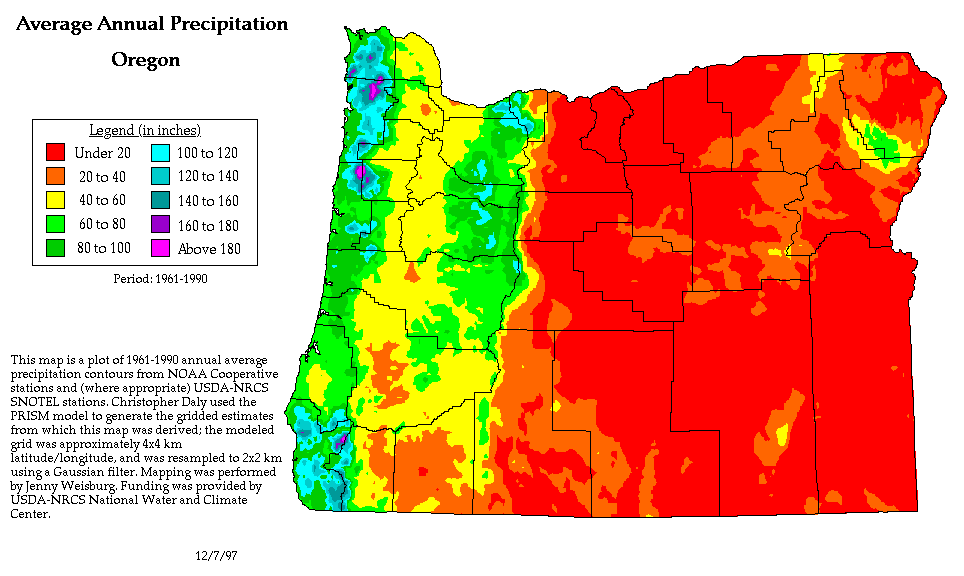 |
We can see the effects of a rain shadow
illustrated well in the state of Oregon. The figure
above at left shows the topography (here's the source
of that map). Winds generally blow from
west to east across the state.
Coming off the Pacific Ocean the winds first encounter a
coastal range of mountains. On the precipitation map
above at right (source)
you see a lot of greens and blue on the western sides of
the coastal range. These colors indicate yearly
rainfall totals that range from about 50 to more than 180
inches of rain per year. Temperate rainforests are
found in some of these coastal locations. The line
separating the green and yellow on the left side of the
precipitation map is the summit, the ridgeline, of the
coastal mountain range.
That's the Willamette River valley, I think, in between
the coastal range and the Cascades. This valley is
somewhat drier than the coast because air moving off the
Pacific has lost some of its moisture moving over the
coastal range.
What moisture does remain in the air is removed as the
winds move up and over the taller Cascades. The
boundary between yellow/green and the red is the
ridgeline of the Cascade Mountains.
Yearly rainfall is generally less than 20 inches per year
on the eastern side, the rain shadow side, of the
Cascades. That's not too much more than
Tucson which averages about 12 inches of rain a year.
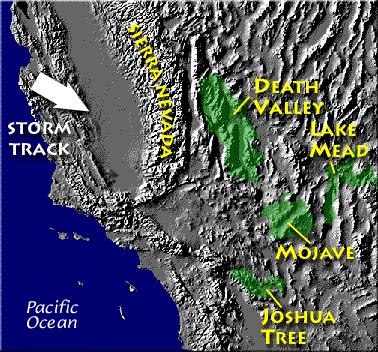
Death valley is
found on the downwind side of the Sierra Nevada
mountains (source of
left image).
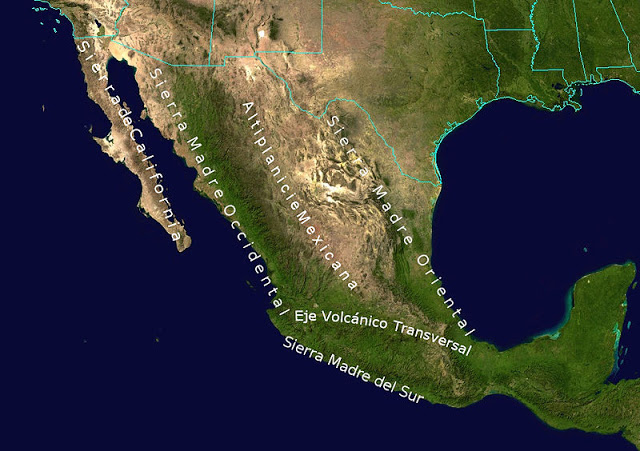
The Chihuahuan desert and the
Sonoran desert are found downwind of the Sierra Madre
mountains in Mexico (source
of the right image).
Mexico might
be a little harder to figure out because moist air can move
into the interior of the country from the east and west.
But there are mountains along both coasts, so some of that
moisture will be removed before arriving in the center of the
county.
Most of the year, the air that arrives in
Arizona comes from the west, from the Pacific Ocean (this
changes in the summer). It usually isn't very moist
by the time it reaches Arizona because it has traveled up
and over the Sierra Nevada mountains in California and the
Sierra Madre mountains further south in Mexico. The
air loses much of its moisture on the western slopes of
those mountains. Beginning in early July in
southern Arizona we start to get air coming from the south
or southeast. This air can be much moister and leads
to development of our summer thunderstorms.
Just as some of the world's driest regions are
found on the downwind side (the rain shadow side) of
mountain ranges, some of the wettest locations on earth
are on the upwind sides of mountains. There seems to
be some debate whether Mt.
Wai'ale'ale in Hawaii or Cherrapunji
India gets the most rain per year. Both get
between 450 and 500 inches of rain per year.