We will now take a look at the energy budget for the planet Earth and provide an explanation for the greenhouse effect. All of the energy into and out of the Earth's climate system is in the form of electromagnetic radiation. A detailed study of this material is too complex to cover in this class. We will go into enough detail to allow you to understand some of the basic properties of radiation and the natural emission of radiation. The laws of radiation will be used to qualitatively describe how the Earth comes to a radiative equilibrium temperature. We will then apply this material to study the global warming issue.
Radiation is a form of energy transfer. Radiation energy emitted (or given off) by one object is delivered to another object when that object absorbs the radiation energy. Radiation energy travels through outer space at the "speed of light" (300,000 km/sec or 186,000 mi/hr). Visible light is just one type or classification for radiation. It is the type of radiation that can be sensed by our eyes. We cannot see other types of radiation, but they exist.
All objects in the universe emit (or give off) radiation. At a very basic level, radiation is emitted photon by photon. Billions upon billions upon billions ... of photons. Different types of radiation can be described by the energy carried by a single photon. As mentioned previously, for studies of the Earth's climate, we are mainly concerned about ultraviolet, visible, and infrared radiation (listed in order from most energetic to least energetic photons). Keep in mind that there exists a continuous spectrum of photon energies in the universe, which extends from much more energetic than ultraviolet to much less energetic than infrared.
For solids, liquids, and high density gases (like stars), we can characterize the radiation emitted using the following two rules:
More specifically the rules above can be written as mathematical equations, that is, given the temperature of an object, the rate of photon emission over the entire range of photon energies can be computed.(See instructive figure of emission curves.) The figure is not drawn to scale because if it were the radiation energy emitted by the Earth would be too small to see next to the much larger radiation energy emitted by the hotter surface of the sun. The figure does show the two radiation laws, though. There are curves for objects at three different temperatures. Although not drawn to scale, the higher temperature objects emit much more total radiation energy (area under each curve) than lower temperature objects. In addition, notice that the peak of each curve, which corresponds to the energy where the object emits most of its photons or radiation, moves toward higher photon energy as the temperature increases. Objects emit radiation energy (photons) over a range of energies around the peak. Objects near the temperature of the Earth's surface are not hot enough to emit any visible radiation and thus can only be seen by reflected light. The heating coil is hot enough to emit some visible radiation. It is mostly the lowest energy visible radiation (the color red), so it is visible to us as glowing red. The sun emits all colors of visible photons and we perceive this mixture as white light. The sun is hot enough to emit some ultraviolet photons, which are potentially harmful to life on Earth. This figure of emission curves for 3000 K to 6000 K bodies shows that the Sun, with a temperature near 6000 K, has its peak emission in the visible radiation range, while cooler objects have peak emissions at lower photon energies. These curves are also drawn on the same scale, which indicates that total radiation energy emitted falls rapidly as the temperature of the emitting object lowers. Here is a figure that shows the emission curves for the Earth and Sun with proper units for radiation energy, which indicates that the hotter Sun, with a temperature near 6000 K, emits over 1 million times more radiation energy per square meter of surface area than the cooler Earth at a temperature of 288 K. The labels below the emission curves indicate that for climate studies, the spectrum of radiation is broken into two components: shortwave radiation (high energy visible and near visible photons from the Sun) and longwave radiation (lower energy infrared photons from the Earth's surface, clouds, and gases in the atmosphere). Shortwave and longwave refer to the wavelength of the electromagnetic radiation, which is not discussed in this class.
The mathematical radiation laws can also be used backwards: by measuring the radiation energy emitted by an object, we can determine its temperature. For example, if I point an infrared detector toward you, I will measure radiation characteristic of a 98°F object. If I point the detector at a colder wall in the room, I will measure less radiation energy, which is the amount characteristic of an object with a temperature of about 75°F. Because the infrared detector is measuring radiation emitted by objects on Earth (rather than normal vision which works by seeing visible light that is reflected from an object), the infrared detector can sense the difference between you and the cold wall even in the dark. This is how passive military night vision works. The presence of people (at night) can be detected by measuring the higher amount of infrared energy emitted by warm bodies compared to colder surroundings. Differences in the infrared energy measured are then displayed as a false color visible images that can be seen by human eyes. The two figures below are false color infrared images. One before the boy bounces a ball and one after the ball has been bounced. Because the ball gets hotter after being bounced it emits more infrared radiation. The scale on the right relates the displayed false color with temperature. Similarly, the temperature of the Earth can be determined by satellite measurements of infrared radiation emitted from the surface of the Earth and the atmosphere.
 |
 |
| False color infrared image of boy before bouncing ball. Temperature color scale shown right of image. | False color infrared image of boy after bouncing ball. |
Remember radiation is one of the ways that energy can be transferred from one object to another. The actual energy transfer takes place when an object absorbs the radiation energy that was emitted by another object. Absorption can be said to occur photon by photon. Once a photon is absorbed, it ceases to exist, and its energy is delivered to that object. In the case of the Earth and Sun, the Earth heats up when it absorbs radiation energy (photons) emitted from the Sun.
Radiation is the only form of energy transfer that can move through the vacuum of outer space. Thus, radiation is the only way for energy to be transfered into or away from the planet Earth. The original source of nearly all of the energy for the physical and biological processes that take place on Earth is radiation energy that came from the Sun. The Earth continuously emits radiation energy off to space. The net effect of these two processes (absorption of radiation energy from the Sun and emission of radiation energy to space) determines the average temperature for the planet.
Because the Sun is much hotter than the Earth, it emits much more radiation energy than the Earth. Since the temperature of the Sun is about 5800 K [5728°C], the majority of the radiation energy it emits is in the form of visible radiation. Similarly, since the temperature of the Earth is about 288 K [15°C], the majority of the radiation energy it emits is in the form of infrared radiation. So the Earth heats up by absorbing mostly visible radiation energy from the Sun (with smaller amounts of energy in the form of ultraviolet and infrared radiation), and cools down by emitting infrared radiation out to space. Over time these two processes come into balance, i.e., radiation energy absorbed equalling radiation energy emitted, and the planet Earth reaches a steady average temperature (as described below):
 |
If there were no atmosphere (and assuming that there were no change in the amount of energy that the Earth absorbs from the sun), the average temperature of the Earth's surface would be equal to the radiative equilibrium temperature of -18°C (0°F). But the atmosphere acts to warm the surface through what is called the atmospheric greenhouse effect. The average surface temperature of the Earth is about 15°C (59°F). We will explain more about the greenhouse effect later. You should understand that the average temperature at the Earth's surface does not have to be equal to the average temperature of the planet as a whole. For instance, we know that the temperature of the atmosphere near the tropopause is much colder than the temperature at the Earth's surface.
Before describing the greenhouse effect, let us take a look at what happens to the radiation from the Sun that hits the planet Earth.
To determine the average temperature of the planet Earth, what is important to know is how much radiation energy from the Sun is absorbed by the planet. The amount of radiation energy that hits the planet can be calculated using the temperature of the Sun, the distance from the Sun to the Earth and the size of the Earth. However, not all of this radiation is absorbed. Some is scattered back to space. Only the absorbed radiation goes into heating the Earth. The following figure illustrates this pictorially.
| We will follow a beam of solar energy and study its interaction with the atmosphere and the Earth's surface. Assume the beam contains 100 units of energy as it enters the atmosphere: |
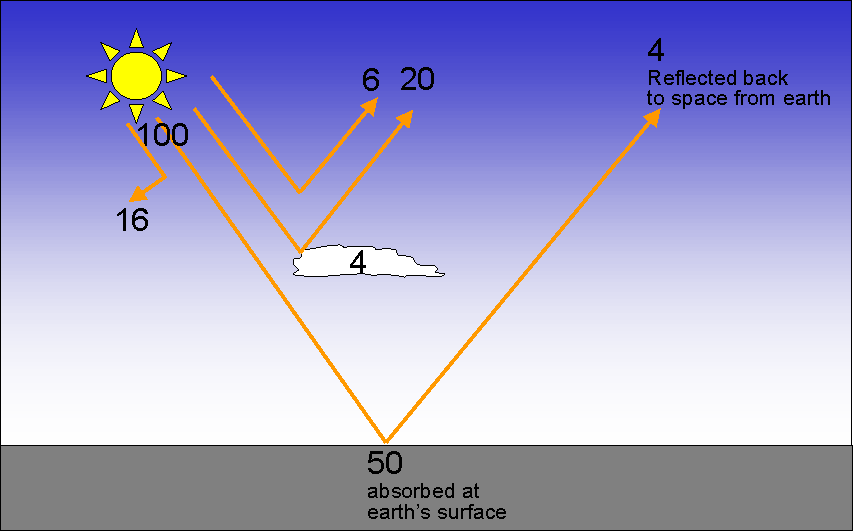
|
| On a worldwide, long-term average, air molecules absorb 16% of the incoming energy from the Sun and reflect another 6%. Clouds absorb 4% of the incoming energy from the Sun and reflect 20%. Therefore 54% of the incoming energy from the Sun is transmitted through the atmosphere and hits the Earth's surface. 4 of the 54 units are reflected from the surface back to space. Therefore, the Earth's surface absorbs 50% of the radiation energy from the sun that hits the planet. |
To summarize this information, of all the solar radiation that hits the planet Earth:
In principle anything that causes a change in the amount of solar energy that the Earth absorbs from the sun should result in a climate change that changes the radiative equilibrium temperature to restore the radiation energy balance. This includes but is not limited to