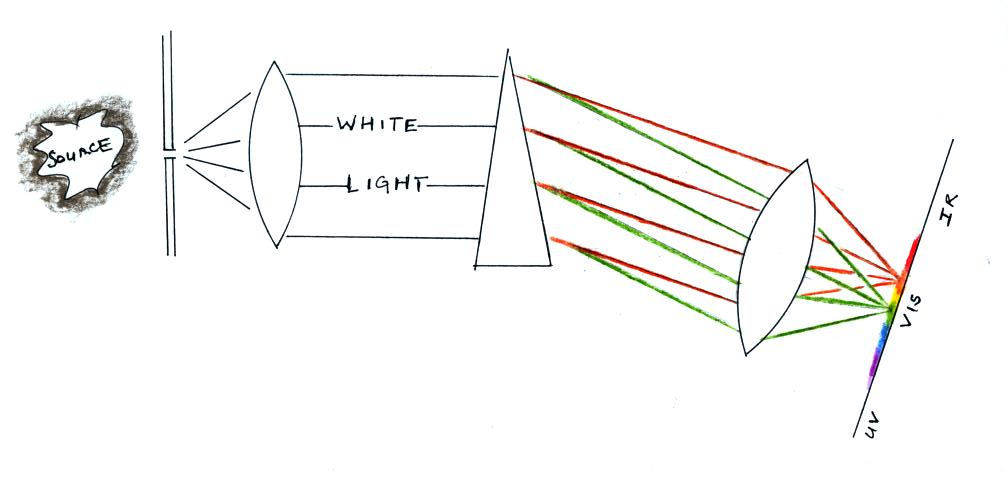
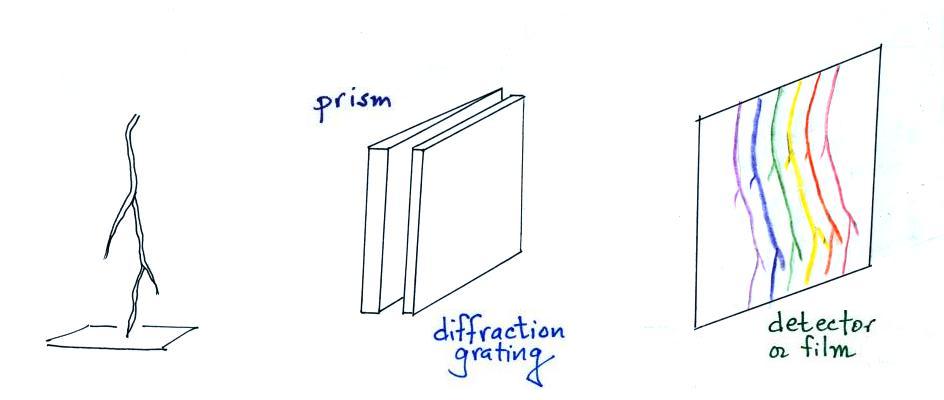
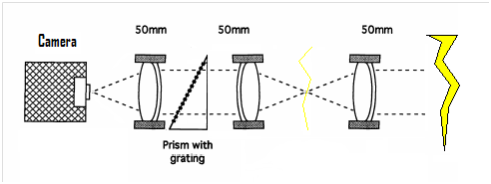
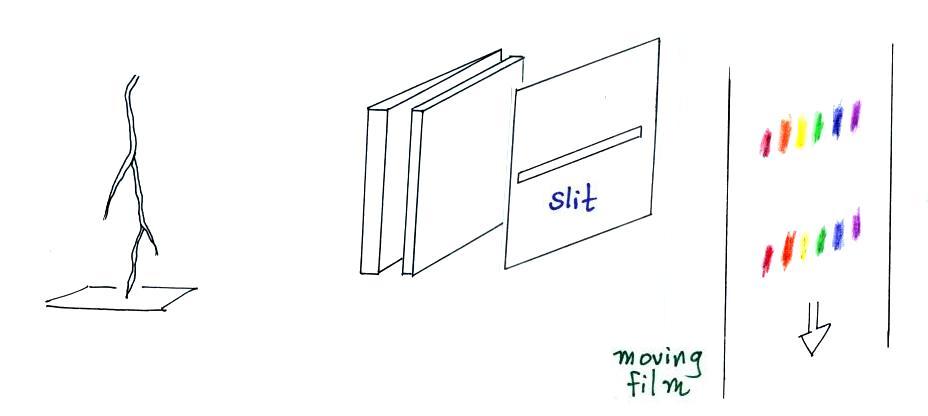
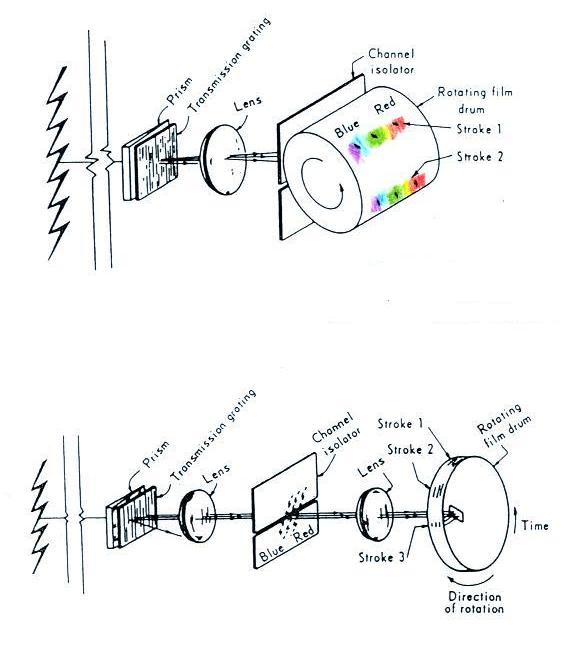
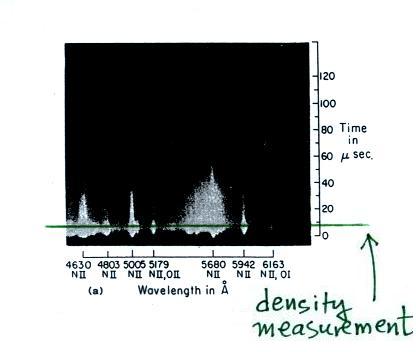
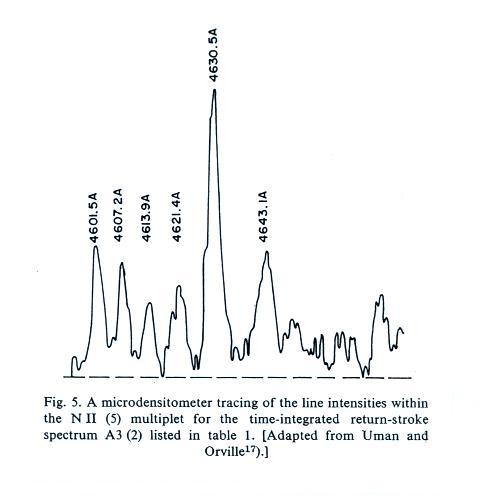
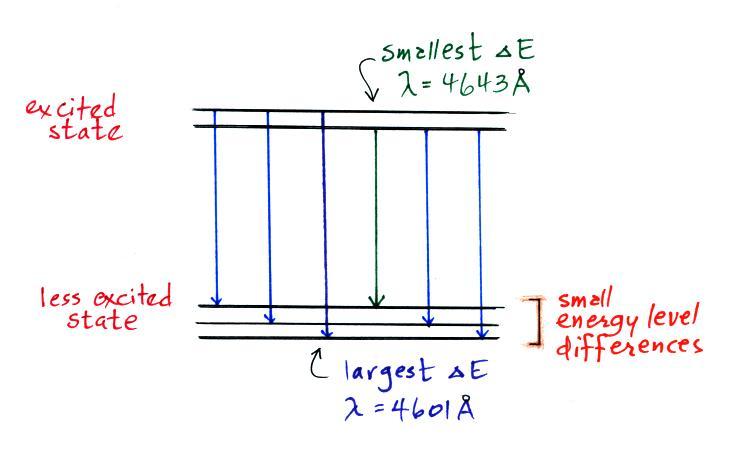
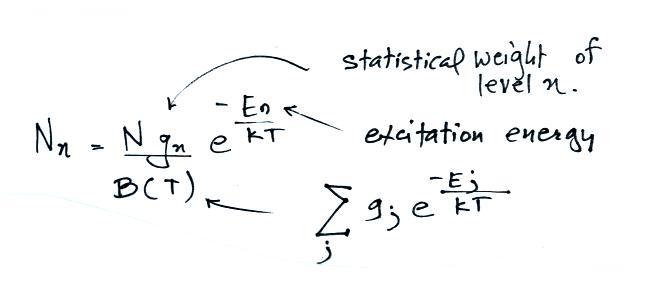
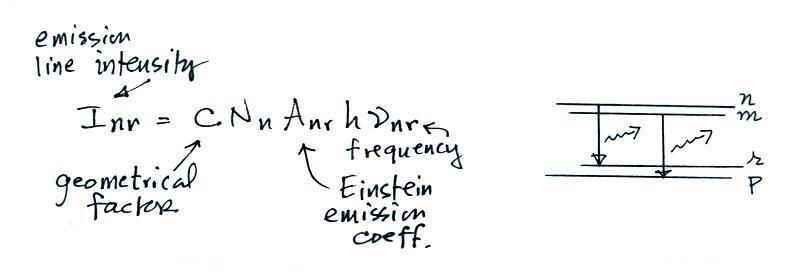
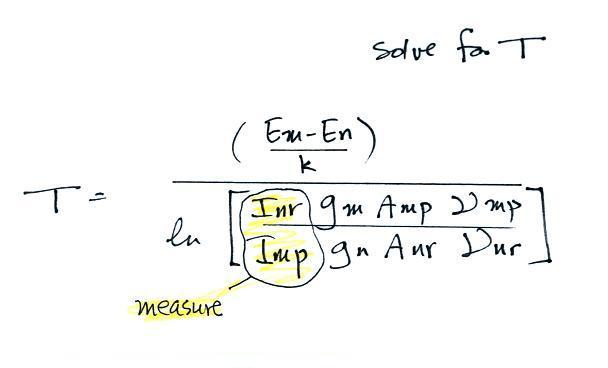
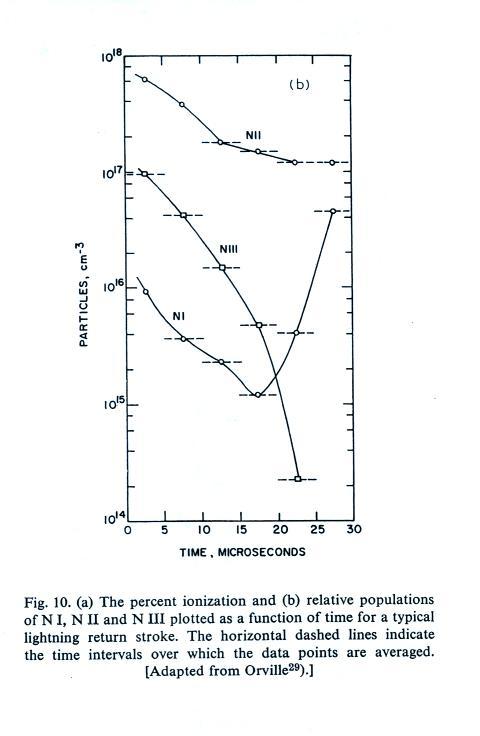
A figure from Cen et al. (2014)
with what may be the first spectrum of ball
lightning (above left). The spectrum of the
ball lightning (BL) can be seen at the very bottom
of the channel. The spectrum above is from
the lightning channel above the ground. Note
that light from the "ball lightning" persists well
after the spectrum from the channel above has
faded. The discharge was 0.9 km
away and the ball lightning emissions persisted
for 1.64 seconds. A somewhat
larger view of the bottom of the lightning channel
and the ball lightning spectrum (from Ball (2014))