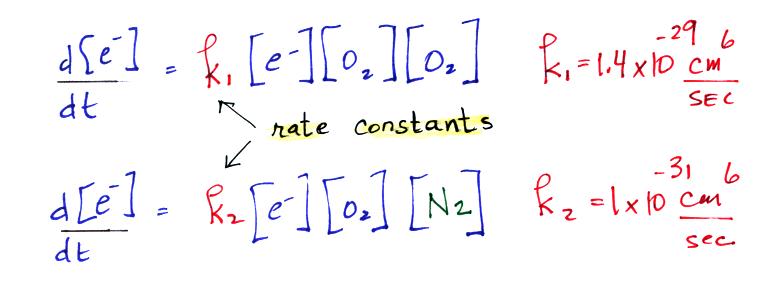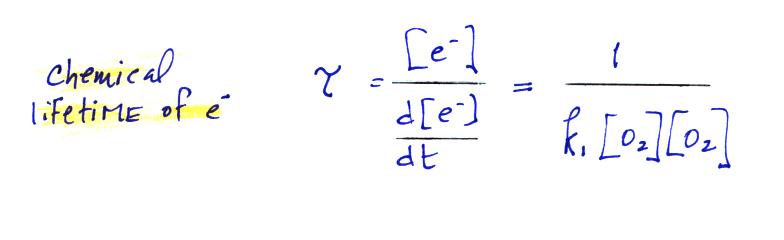Here are some more details concerning the attachment of free
electrons to oxygen molecules. Time constraints often keep
us from covering this in class.
When neutral oxygen or nitrogen are ionized you are left with a
positively charged N2 or O2 molecule and a free electron.
The electron subsequently attaches to neutral oxygen molecules
(but not to nitrogen). The time that this takes can be
calculated in a relatively straight forward way. The
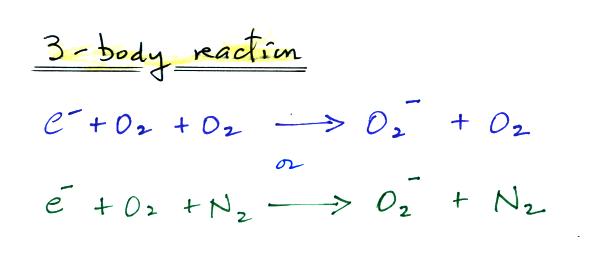
electron attachment is described with a "3-body" reaction
equation.
From what I learned here, the
electron and two oxygen molecules don't collide
simultaneously. Rather an electron and an oxygen molecule
collide and produce an "energetically excited reaction
intermediate" which then collides with a different oxygen
molecule that carries off the excess energy.
The corresponding reaction rate equations are (the
[square brackets] denote concentration)
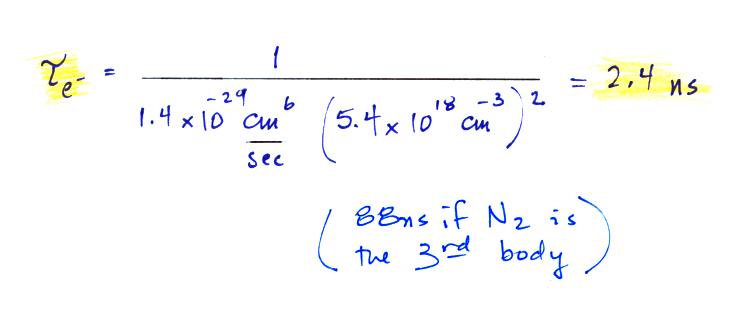
The average lifetime of a free electron is given by the
following expression
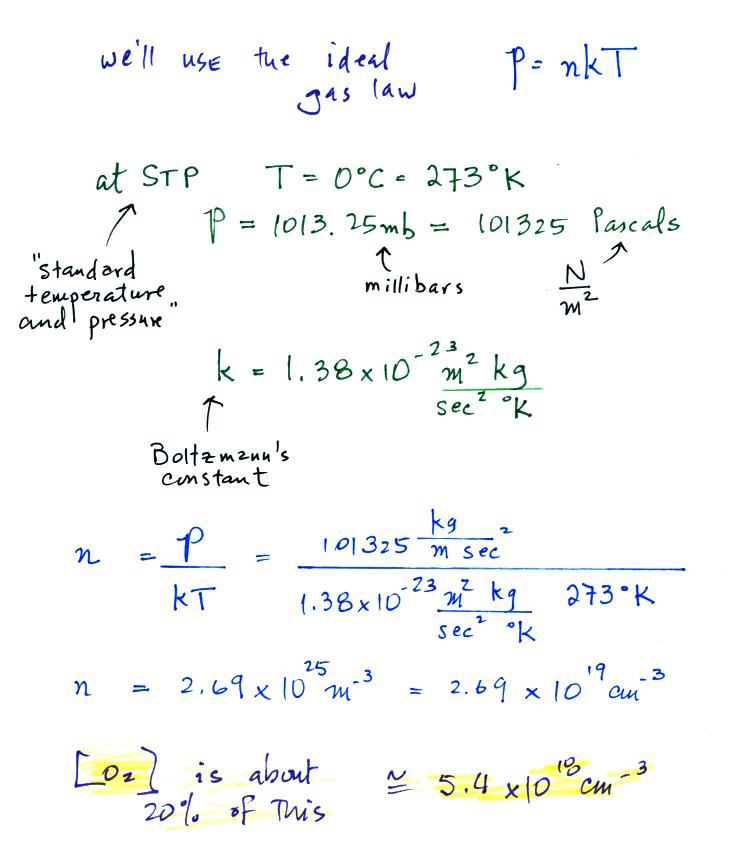
We have the rate constant k1 but we also
need to know the oxygen concentration in air, [O2].
That's something we can calculate using the ideal gas law.
Electron attachment occurs very quickly, in a few or a few 10s of
nanoseconds.
The attachment time is very short when the oxygen and nitrogen
concentrations are high. The time gets longer higher in the
atmosphere where [O2] and [N2] are lower.