Tuesday Oct. 3, 2006
Optional Assignment #2 was collected in class today.
Optional Assignment #3 questions will be asked
during class on Tuesday and Thursday this week. This
assignment will be due at the beginning of class next Tuesday (Oct. 10).
The Experiment #2 reports and the Expt. #1 report revisions are also
due next Tuesday (Oct. 10). Return your Expt. #2 materials this
week so that you can pick up the supplementary information sheet.
All of the 1S1P Assignment #1
papers have now been graded.
Have a quick look at the classnotes from last Thursday's class. A few things were
slipped in after
class. A figure explaining the difference between temperature (a
measure of the average kinetic energy) and heat (the total kinetic
energy of the atoms or molecules in an object) was added. You
should also know the boiling points and freezing points of water on the
Fahrenheit, Celsius, and Kelvin temperature scales (the freezing point
of water is equal to the melting point of ice). 300 K is a good
easy to remember value for the average temperature of the surface of
the earth.
There are
four energy transport processes: conduction, convection, latent heat
and electromagnetic radiation. We'll look at conduction first; in
the atmosphere it is the least important of the four.

The figure above illustrates energy transport by
conduction. A
hot object is stuck in the middle of some air. In the first picture the
random motions of the atoms or
molecules near the object have caused them to collide with and pick up
energy from the object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red). In the middle picture the
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object. In
the third picture molecules further from the object now have gained
some energy (the yellow ones). The random motions and collisions
between molecules
is carrying energy from the hot object out into the material.
The rate of energy transport depends on the material. Thermal
conductivities of some materials are listed above. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors. Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
Here's a demonstration that we won't be able to do in
class.
The demonstration would involve opening a of glacial acetic acid
(acetic
acid gives vinegar its characteristic smell) in the front of the
classroom. The acetic acid would begin to evaporate into the
air. Collisions with air molecules would begin to move the acetic
acid molecules toward the back of the room.
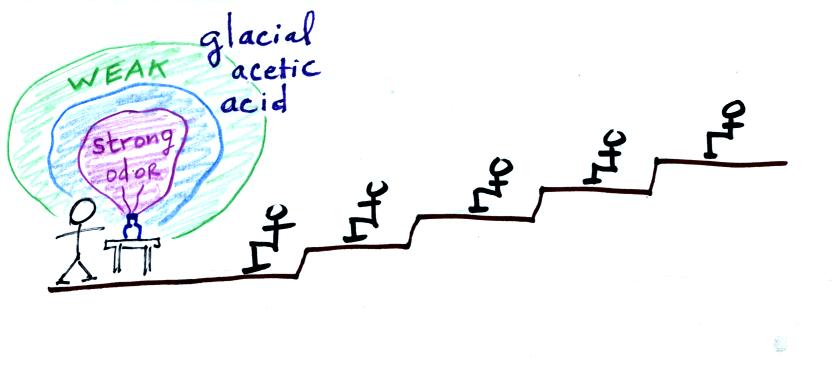
The strong irritating odor of the acetic acid would make it
difficult
to breath at the front of the room.

The odor would eventually spread throughout the class
room. This is an example of diffusion. Because it involves
random molecular motions it is, in many respects, like the conduction
of
heat.
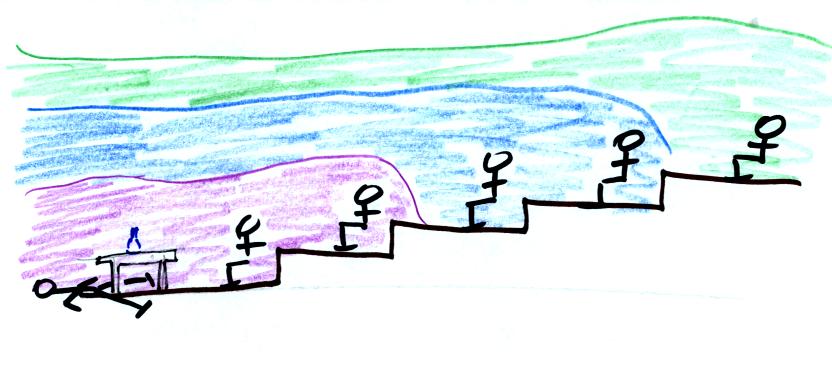

Convection is a second way of transporting energy.
Convection
involves more organized motion of atoms or molecules in a liquid or gas
(but not in a solid, the atoms or molecules aren't able to move freely
enough).
In the top picture above the air surrounding a hot object has been
heated by conduction. Then a person (yes that is a drawing of a
person's head) is blowing the blob of warm air
off to the right. Cooler air moves in and surrounds the hot
object and the cycle can repeat itself. This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly.
Note, in the bottom left figure, that the hot air is also low density
air. It actually isn't necessary to blow on the hot object, the
warm air will rise by itself. Energy is being transported away
from the hot object. This is called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands (as it
moves into lower pressure surroundings) and cools. If the air is
moist, clouds can form.
Note the example at right is also free convection. The sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.
Next are
some real world applications of heat transport by conduction and
convection
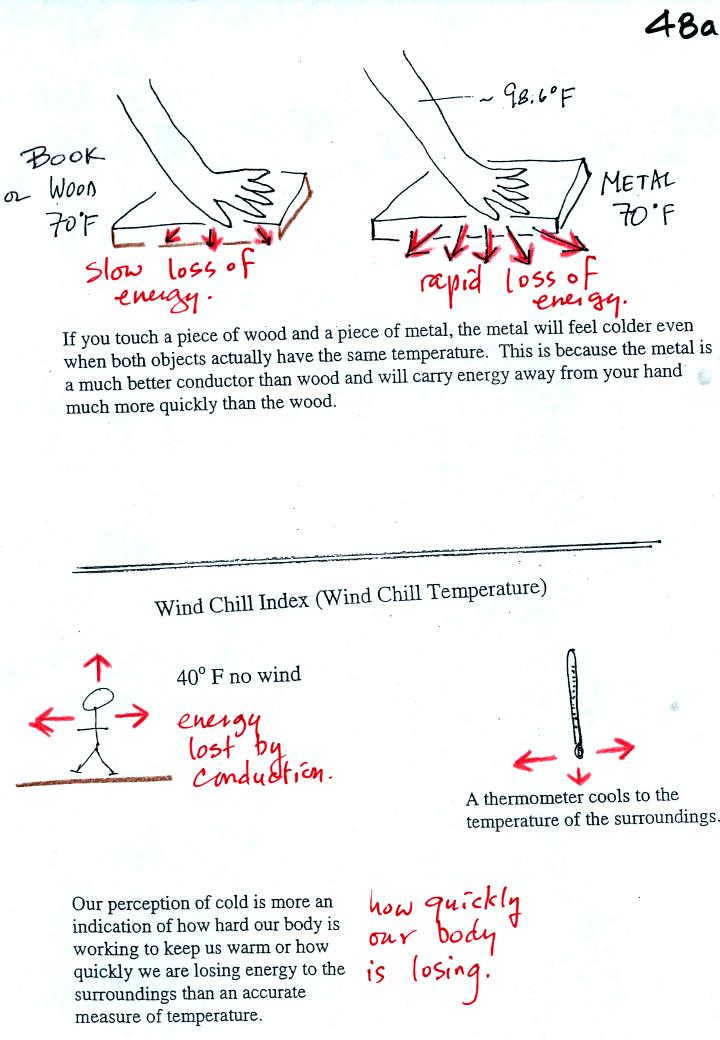
Metals are better conductors than wood. If you touch a
piece of
70 F metal it will feel colder than a piece of 70 F wood. A piece
of 70 F diamond would feel even colder because it is a better conductor
than metal. Our perception of cold is more an indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
Touching a piece of ice also feels colder even though ice is not an
especially good conductor. The cold feeling tells us that our
hand is losing a lot of energy. I this case the high rate of
energy loss is due to the large temperature differrence between our
hand and the ice rather than just the thermal conductivity of the ice.
If you go outside on a 40 F day (calm winds) you will feel cold; your
body is losing energy to the colder surroundings. A thermometer
behaves differently. It actually cools to the temperature of the
surroundings. Once there it won't lose any additional energy.
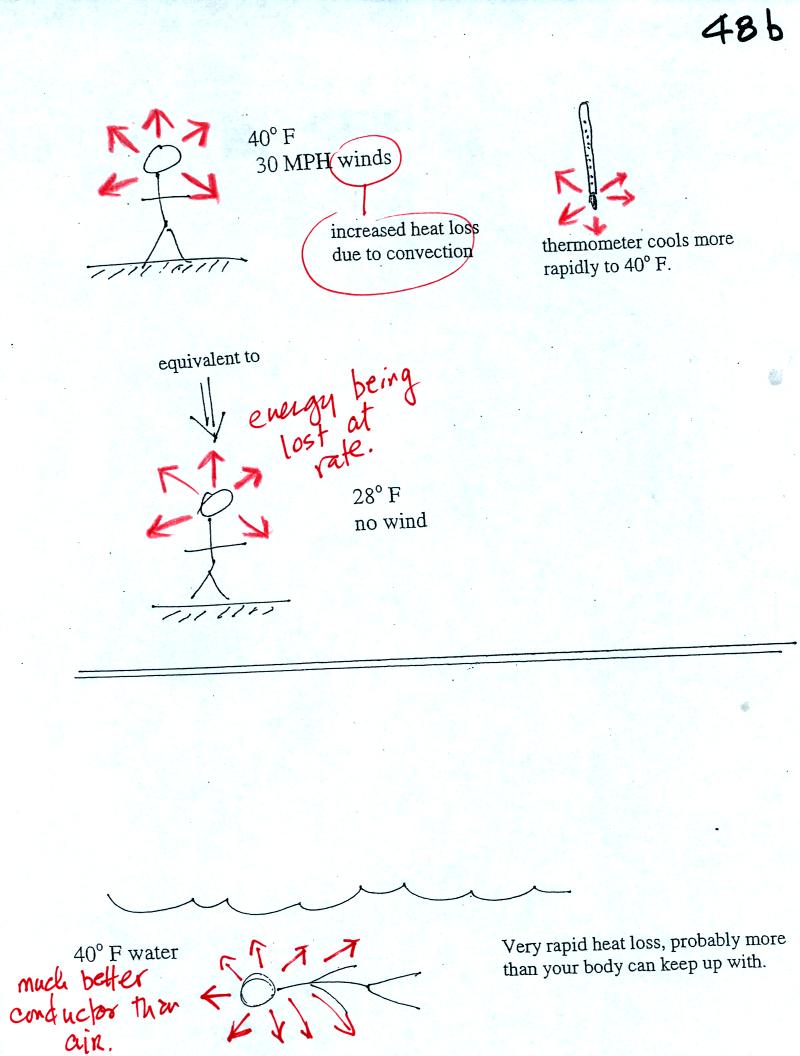
If you go outside on a 40 F day with 30 MPH winds your body
will lose
energy at a more rapid rate. It will feel colder than a 40 F day
with calm winds. Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a calm 28
F day. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F. The thermometer will again cool to the
temperature of its surroundings. ON a windy day it will cool more
quickly, but once it ends up at 40 F it won't cool any further. The
thermometer would measure 40 F on both the calm and the windy day.
Water is a much better conductor than air. If you fall into 40 F
water your body will lose energy at a high enough rate that your
metabolism might not be able to keep up with it. Falling into 40
F water is a life-threatening situation.
Energy
transport in the form of latent heat is the second most important
energy transport process (second only to electromagnetic
radiation). It is a little tricky to see how the energy is
actually transported from one place to another. Before worrying
about that a little review is necessary.

A solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously supply the energy (such as when you put water in a
pan and put the pan on a hot stove) or the needed energy will be
taken from the surroundings (from your body when you step out of a
shower in the morning).
Here's a school kids analogy:
You need to give a kid some energy in order to get him or
her up and
walking around. Even more energy is needed to get the kid outside
running.
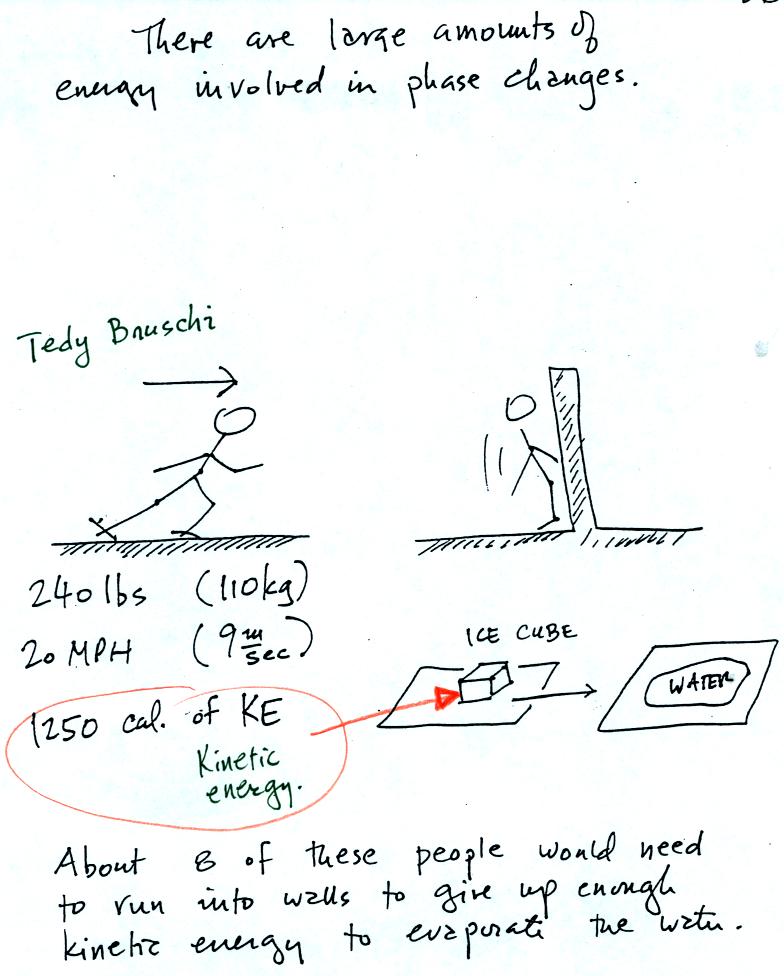
A 240 pound man (or woman) running at 20 MPH has just enough
energy to
be able to melt an ordinary ice cube. It would take 8 people to
evaporate the resulting water.

You can consciously remove energy from water vapor to make
it condense
or from water to cause it to free (you could put water in a freeze, a
cold "box"; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm).
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more rapidly.
Here are the school kids again. They're out on the play ground
running around and you need to get them back inside the classroom and
sitting at their desks.
Now we put
everything together and see how energy gets carried
from one place to another.

The story starts at left in the
tropics where there is often an abundance or surplus of energy;
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.
We'll
spend the next couple of class periods on electromagnetic
radiation. It is the most important energy transport process
because it can travel through empty space.
To really understand EM radiation you need to understand electric
fields. To understand electric fields we need to quickly review
static electricity.
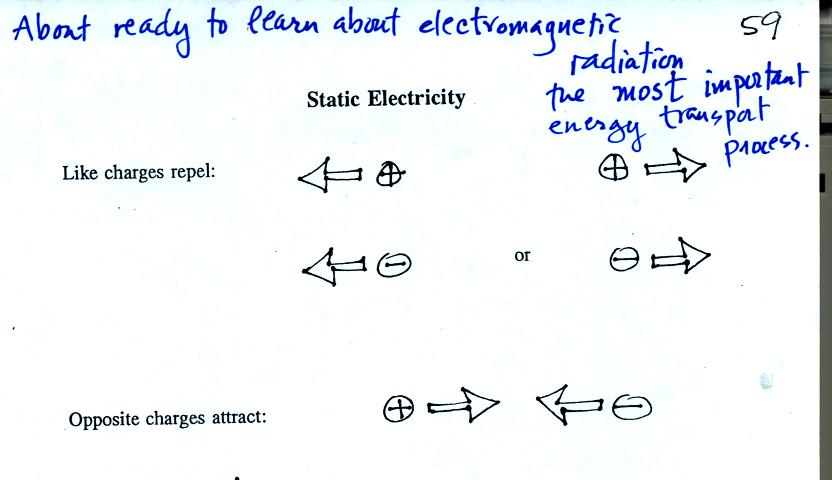
We used a sweater and two balloons to demonstrate
the rules above.
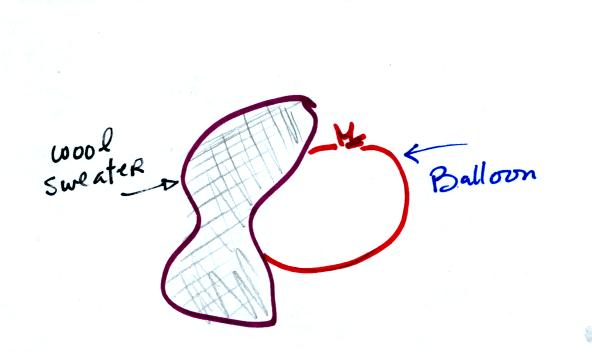
If you rub a balloon with a wool sweather the balloon and
the sweater
become electrically charged (static electricity is one of the reasons I
don't like wearing wool sweaters).
We actually charged up two balloons. We didn't know what charge
the balloons carried just that they both had the same charge.
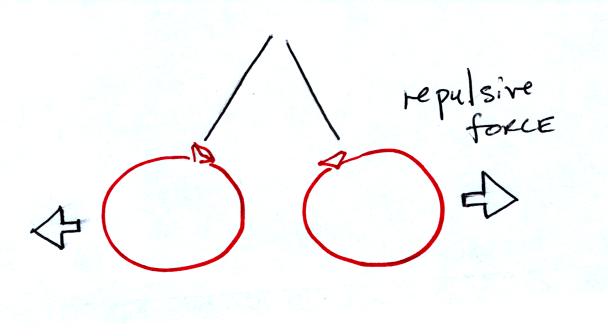
If you bring the balloons close to each other they are
pushed apart by
a repulsive electrical force.
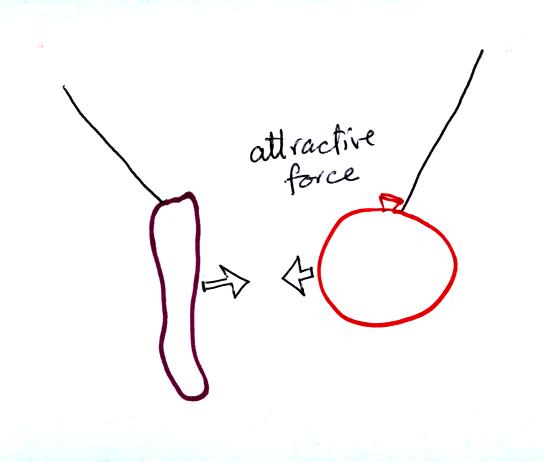
The sweater and the balloon carry opposite charges. IF
they are
brought together they experience an attractive electrical force.
Now the bottom figure from p. 59 in the photocopied class notes.
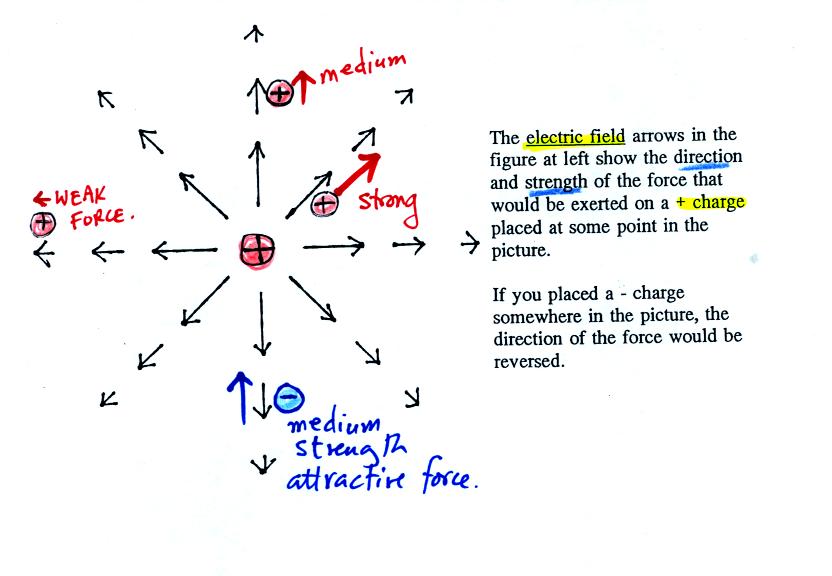
The balloons can help you understand
the picture above. Imagine placing one of the balloons at the
center of the picture and assume that it is positively charged.
The second balloon is placed at various positions (1, 2, and 3) around
the central balloon. The arrows in the picture are the electric
field. They give the direction and strength of the force that
would be exerted on the second positive charge. At Position 1,
for example a positively charged balloon would be pushed (by the +
charge on the center balloon) to the upper right with a strong
force. At Position 2 the force points straight up but isn't as
strong because the + charge at Position 2 is further from the center
charge. At Position 3 the charge is pushed to the left with a
weak force.
You can also use the electric field arrows to figure out what would
happen to a negative charge. The direction of the force is
reversed.
Here are some sample questions about static
electricity and electric fields (these are not part of Optional
Assignment #3).
The
figures on p. 60 in the photocopied class notes have
been broken into 3 parts
below for clarity.
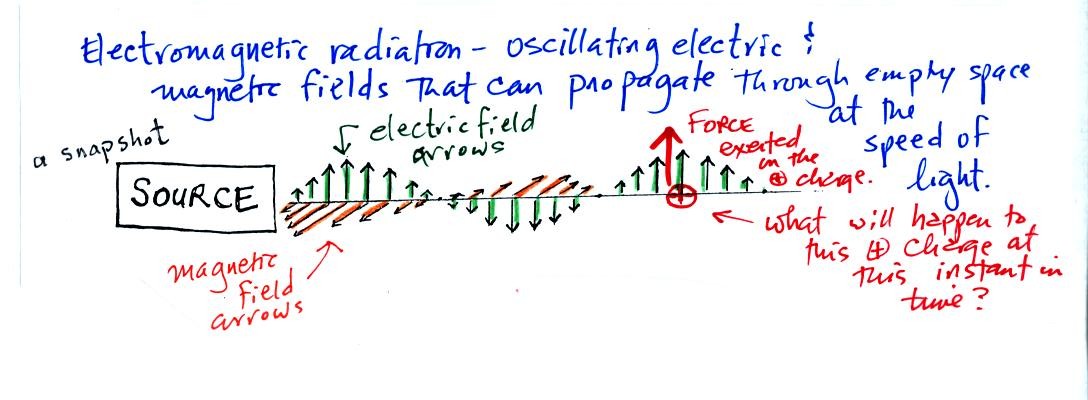
We imagine turning on a source of EM radiation and then a
short time
later we take a snapshot. The EM radiation is a wavy pattern of
electric and magnetic field arrows. We'll ignore the magnetic
field lines. The E field lines sometimes point up, sometimes
down. The pattern of arrows repeats itself.
Note the + charge near the right of the picture. At the time this
picture was taken the EM radiation exerts a fairly strong upward force
on the + charge.
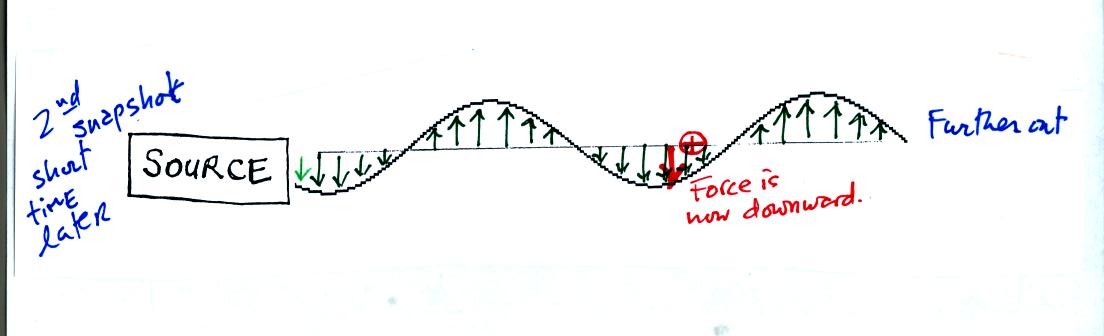
This picture was taken a short time later and the radiation has
traveled a little further to the right. The EM radiation now
exerts a relatively weak downward force on the + charge.

The + charge is now being pushed upward again. A movie of
the +
charge would show it bobbing up and down much like a swimmer in the
ocean would do as waves passed by.
The wavy pattern used to
depict EM radiation (the wavy line connects the heads of the electric
field arrows) can be described spatially in terms of its
wavelength,
the distance between identical points on the pattern.
Or you can
describe the radiation temporally
using the frequency of oscillation
(number of up and down cycles completed by an oscillating charge per
second)




















