Friday Aug. 31, 2007
The first 1S1P assignment of the semester
was mentioned and briefly discussed.
Meanwhile local weather forecasters are keeping an eye on Tropical
Storm Henriette (the storm may have dissipated and disappeared from
the National Hurricane Center by
the time you click on the link at left). Some models show
moisture from that storm system making its way up into southern Arizona
toward the end of next week. This is a possibility not certainty
or even likely at this point.
We first
finished the plot of temperature versus altitude and the discussion of
the troposphere and stratosphere that was started in class last
Wednesday. You'll find all of that in the Wed.,
Aug. 29 class notes.
Sulfur dioxide was discussed briefly in class on Wednesday.
Sulfur dioxide is one of the pollutants that can react with water in
clouds to form acid rain. The formation and effects of acid rain
are discussed on p. 12 in the photocopied Class Notes.

Note that clean unpolluted rain has a pH less than 7 and is
slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. Acid rain is often a problem in regions that are
100s even 1000s of miles from the source of that sulfur dioxide that
forms the acid rain.

Some of the problems or consequences of acid rain.
A short colorful and calming acid rain
demonstration was done in class. Carbon dioxide gas was used
instead of sulfur dioxide.
What
follows is a little more detailed
discussion of the basic concepts of mass, weight, and density
(found on p. 23 in the photocopied Class Notes) than was done in class.
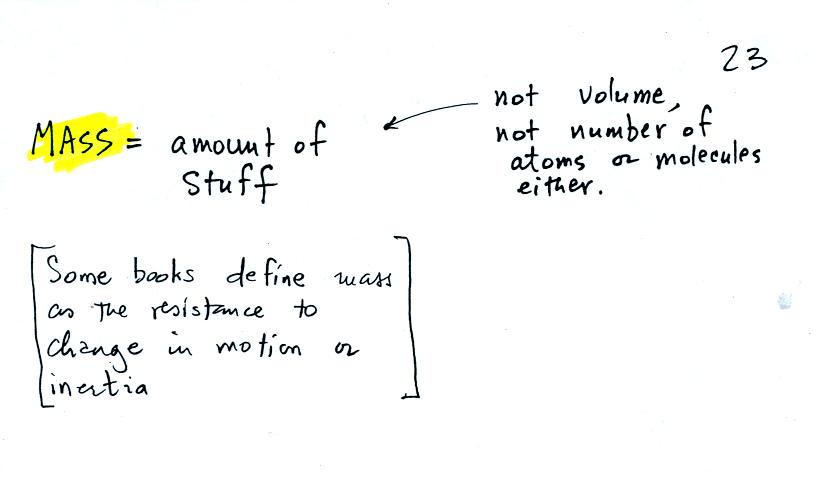
Before we can learn about atmospheric pressure, we
need to review
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
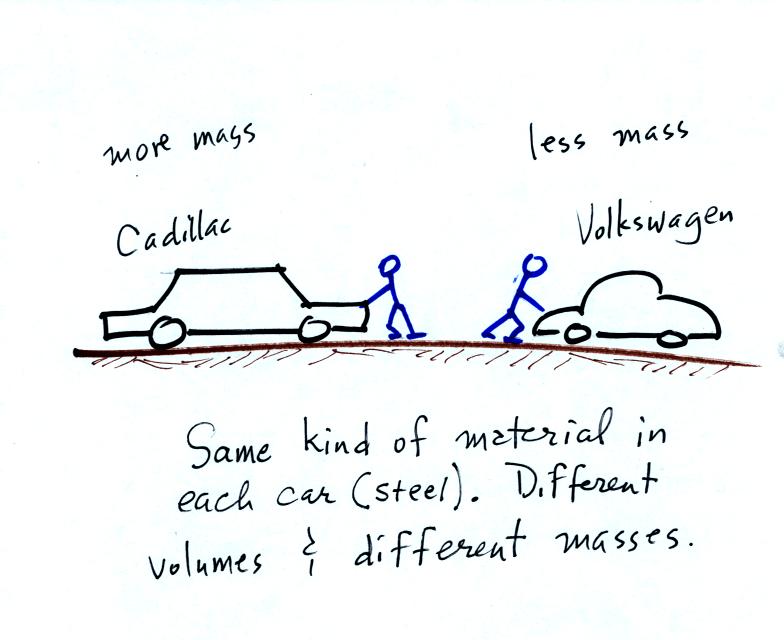
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down if it were
already moving).
It is possible to have two objects with the same
volume but very
different masses. Here's an example:

Bottles containing equal volumes
of water and mercury were
passed around in class (thanks for being careful with the bottles of
mercury). The bottle of mercury was quite a bit heavier than the
bottle of water.
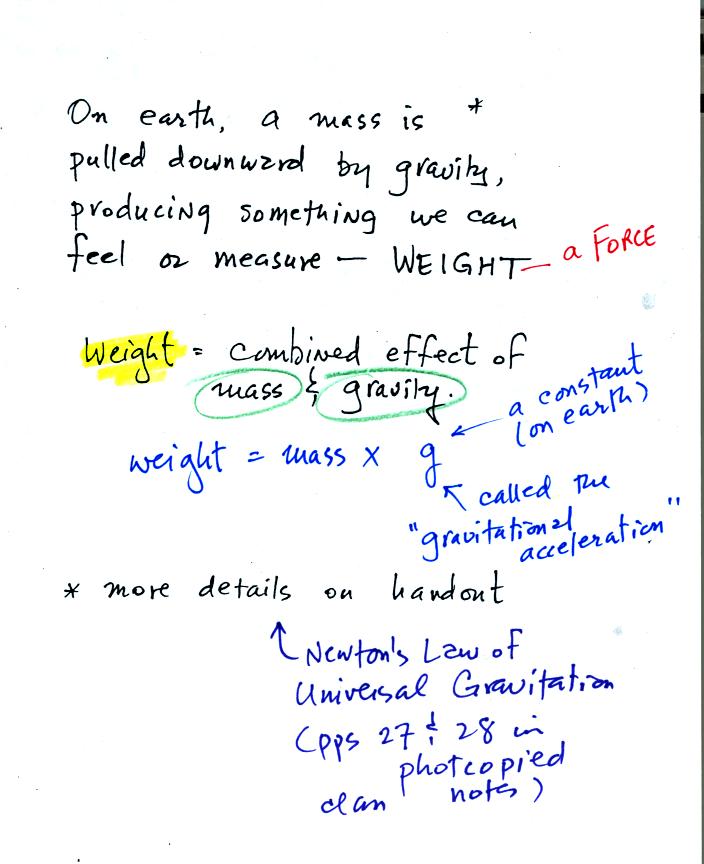
Weight is a force and depends on
both the mass of an object and the
strength of gravity.
We tend to use weight and mass interchangeably
because we spend all our
lives on earth where gravity never changes.
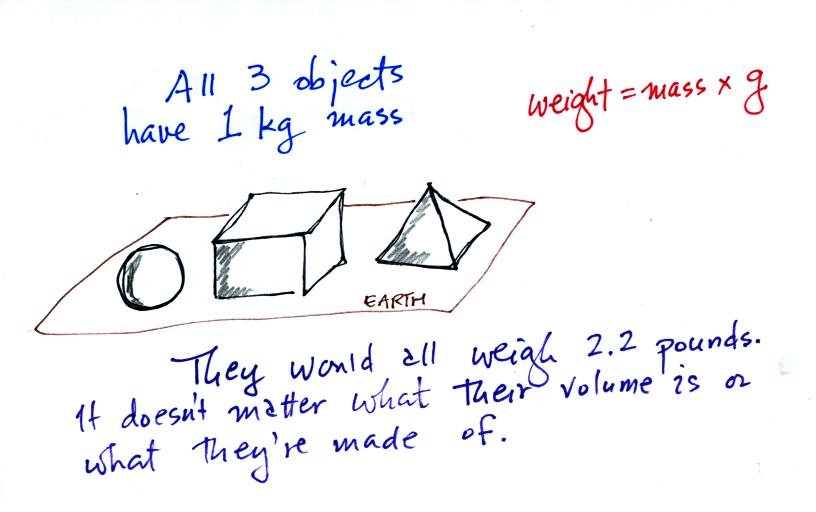
Any three objects that all have the same mass
would
necessarily have the same weight. Conversely
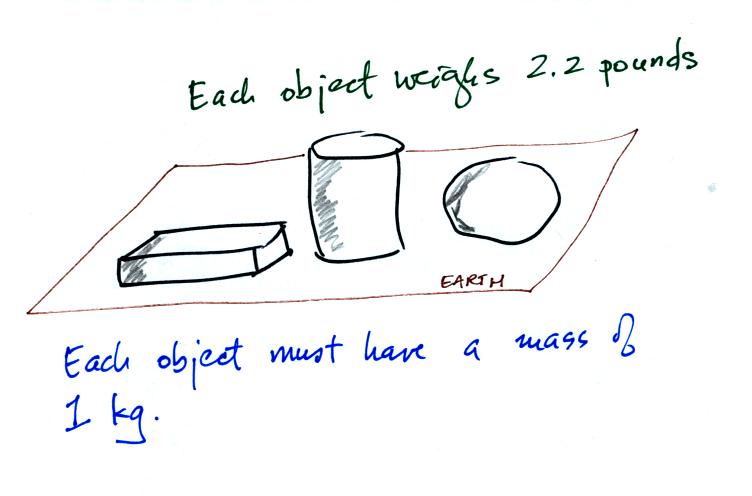
Three objects with the same weight
would have the same mass.
The difference between mass and weight is clearer (perhaps) if you
compare the situation on the earth and on the moon.
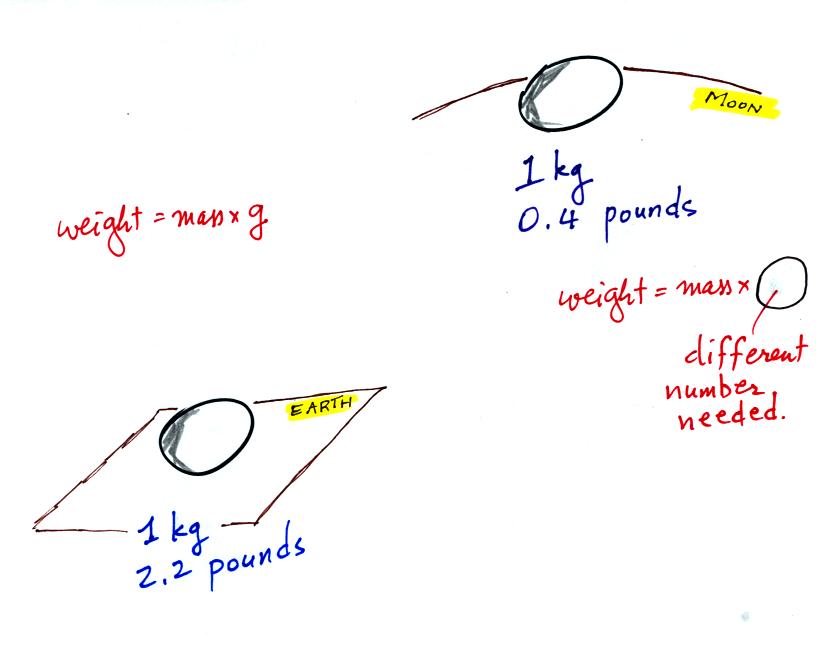
If you carry an object from the
earth to the moon, the mass
remains the
same (its the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.
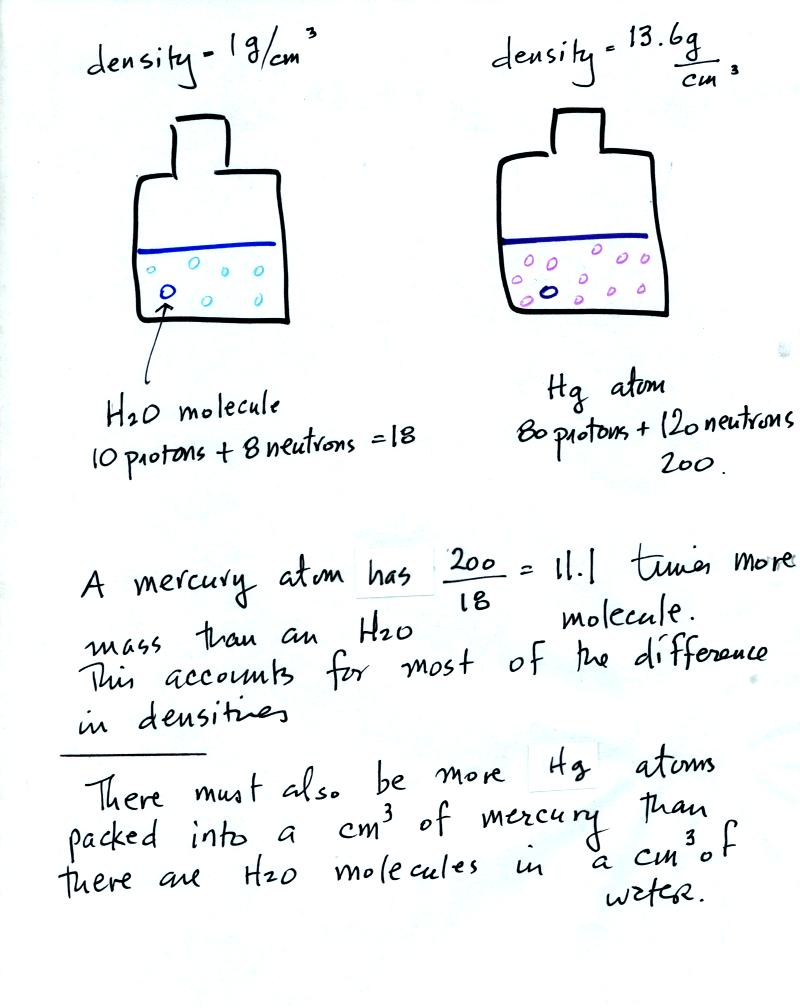
Mercury atoms are built up of many
more protons and neutrons
than a water molecule (also more electrons but they don't have nearly
as much mass as protons and neutrons). The mercury atoms have
11.1 times as much mass as the water molecule. This doesn't quite
account for the 13.6 difference in density. Despite the fact that
they contain more protons and neutrons, the mercury atoms must also be
packed closer together than the molecules in water.
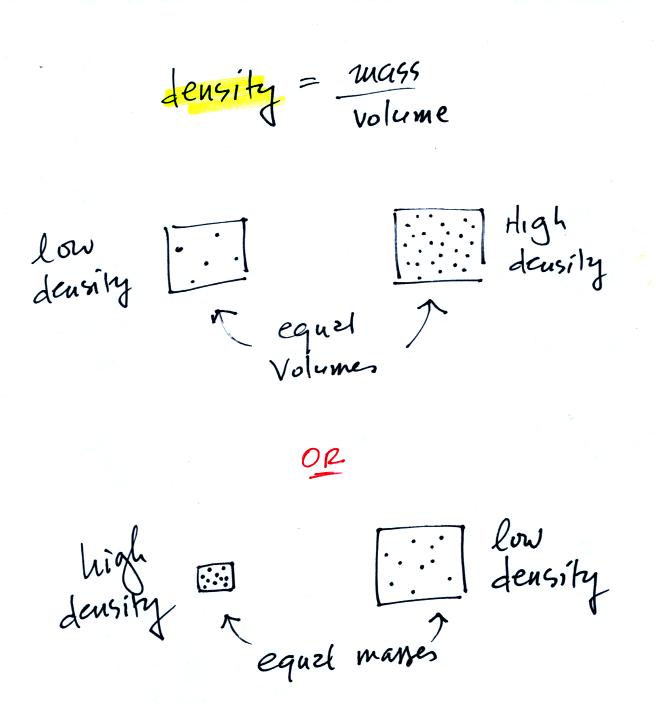
Definition and illustrations of
high and low density.
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo used a simple
experiment to prove that air has weight.
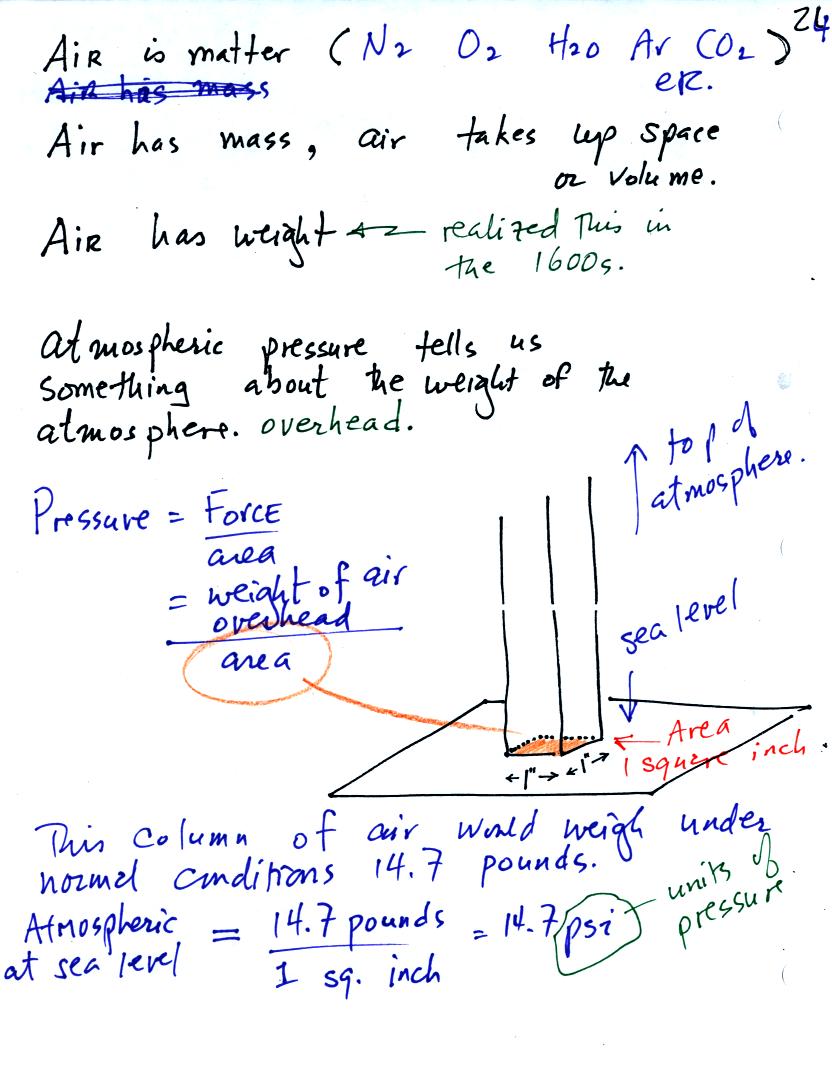
Pressure is defined as force divided by area. Air pressure is the
weight
of the atmosphere overhead divided by area the air is resting on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead.
Under normal conditions a 1 inch by 1 inch column of air stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your car or bike tires with air).
We'll return to this figure in class next Wednesday or Friday and learn
about the millibar units most commonly used by meterologists for
atmospheric pressure.












