Monday Oct. 1, 2007
The 2nd optional assignment was collected in class today. A new
optional assignment was handed out. It will be due at the
beginning of class next Monday (Oct. 8). This new optional
assignment will give you some practice with questions on
electromagnetic radiation.
The Experiment #2 reports and the revised
Expt. #1 reports are both due next Monday. Experiment #2 doesn't
take long to perform, you should plan on returning the materials this
week so that you can pick up the supplementary information sheet.
About 2/3rds of the 1S1P Assignment #1 reports have been graded and
were returned in class today.
The sun
emits electromagnetic radiation. That shouldn't come as a surprise
since you can see it and feel it. The earth also emits
electromagnetic radiation. It is much weaker and invisible.
The kind and amount of EM radiation emitted by the earth and sun depend
on their respective temperatures.
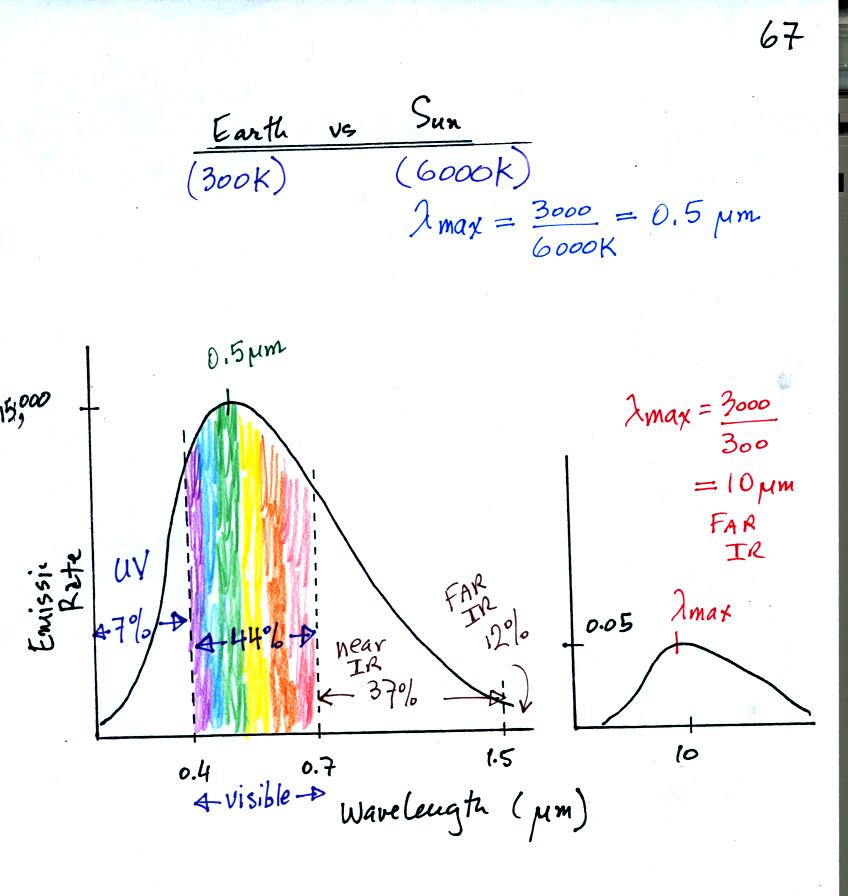
The curve on the left is for the sun. We first used Wien's
law and a temperature of 6000 K to calculate lambda max and got
0.5 micrometers. This is green light; the sun emits more green
light than any other kind of
light. The sun doesn't appear green because it is also emitting
lesser amounts of violet, blue, yellow, orange, and red - together this
mix of
colors appears white. 44% of the radiation emitted by the sun is
visible light, 49% is IR light (37% near IR + 12% far IR), and 7%
is ultraviolet light. More than half of the light emitted by the
sun is invisible.
100% of the light emitted by the earth (temperature = 300 K) is
invisible IR light. The
wavelength of peak emission for the earth is 10 micrometers.
Because the sun (surface of the
sun) is 20 times hotter than the earth a square foot of the sun's
surface emits energy at a rate that is 160,000 times higher than a
square foot on the
earth. Note
the vertical scale on the earth curve is different than on the sun
graph. If both the earth and sun were plotted with the same
vertical scale, the earth curve would be too small to be seen.
We now
have most of the tools we will need to begin to study energy balance on
the earth. It will be a balance between incoming sunlight
energy and outgoing energy emitted by the earth. We will look at
the simplest case, first, the earth without an atmosphere (or at least
an atmosphere without greenhouse gases)
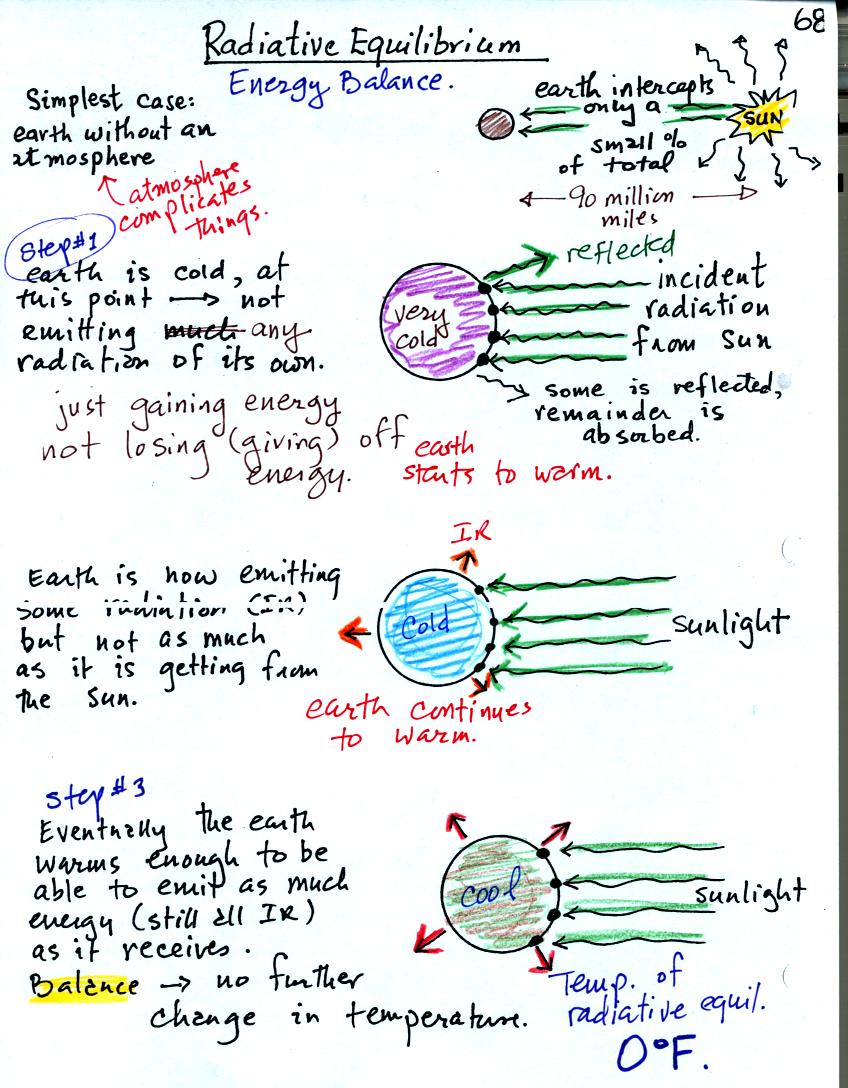
You might first wonder how, with the sun emitting so much more
energy than the earth, it is possible for the earth to be in energy
balance with the sun. The earth is located about 90 million miles
from the sun and therefore only absorbs a very small fraction of the
energy emitted by the sun.
To understand how energy balance occurs we start, in Step #1, by
imagining that the earth starts out very cold and is
not emitting
any EM radiation at all. It is absorbing sunlight however so it
will
begin to warm.
Once the earth starts to warm it will also begin to emit EM
radiation, though not as much as it is getting from the sun (the
slightly warmer earth in the middle picture is now colored blue).
Because the earth is still gaining more energy than it is losing the
earth will warm some more.
Eventually it will warm enough that the earth (now shaded greenish
brown) will
emit the same amount
of energy (though not the same wavelength energy) as it absorbs from
the sun. This is radiative equilibrium, energy balance. The
temperature at
which this occurs is 0 F.
That is called the temperature of radiative equilibrium. You
might remember this is the figure for global annual average surface
temperature on the earth without the greenhouse effect.
Next we
are going to see that the atmosphere behaves somewhat differently, when
it comes to emitting and absorbing electromagnetic radiation, than the
earth or the sun. We will need to understand, in particular, how
the atmosphere filters incoming sunlight and outgoing IR radiation
emitted by the ground.
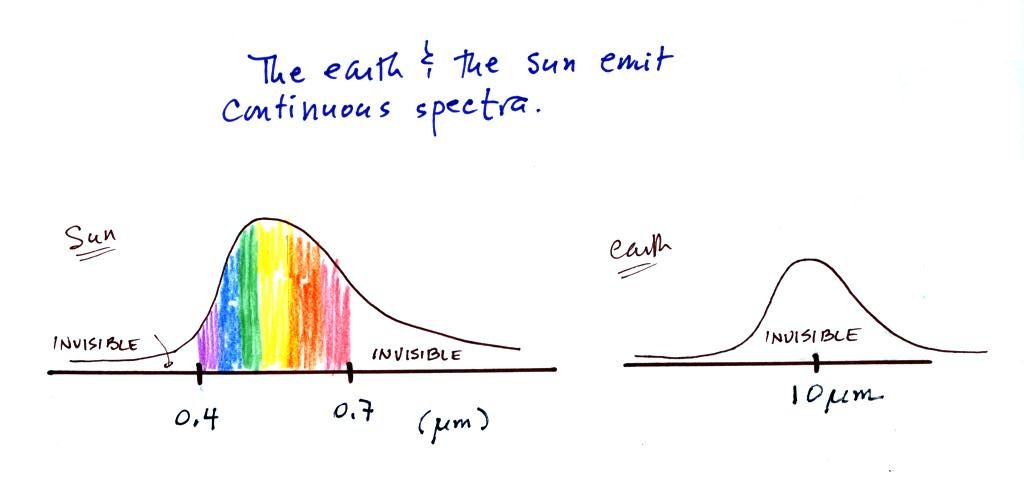
In some respects the earth and the sun behave just like the
tungsten filament in the light bulb used in the class
demonstration. Both the earth and the sun emit continuous
spectra. Part of the light emitted by the sun falls in the
visible part of the spectrum. If you were to look at the sun
(something you shouldn't do of course) with one of the diffraction
gratings handed out in class you would see all the colors of visible
light. There wouldn't be any gaps or colors missing. This
is shown again at the top of the figure below.
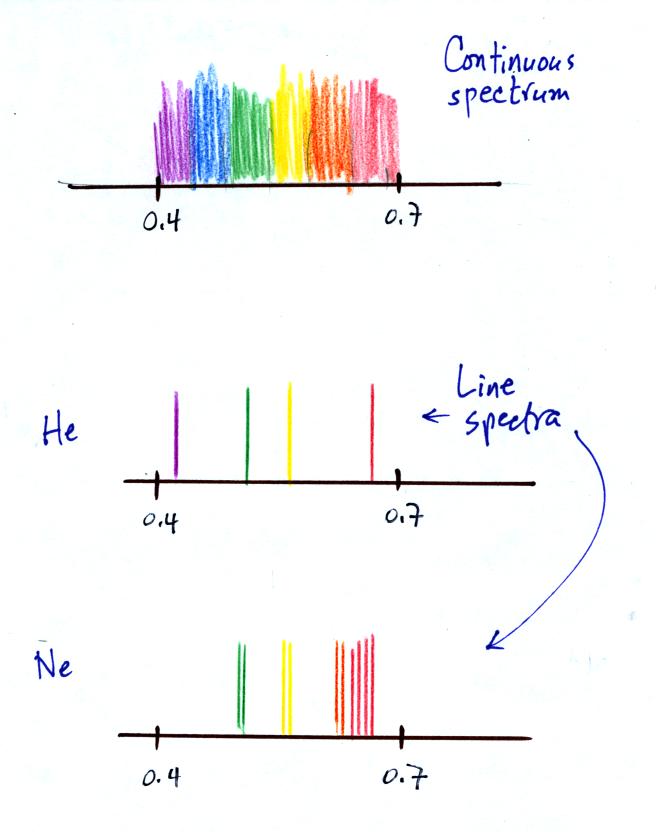
Gases behave differently. We looked at the visible light
emitted by helium (a high-voltage power supply was need to heat the
helium so that it would be hot enough to emit visible light).
Rather than a continuous spectrum, the helium emitted only certain
wavelengths. This is called a line spectrum. Helium
contains only two electrons and is a relatively simple atom and its
line spectrum was also fairly simple.
Neon (Ne) also emitted a line spectrum, thought there were more lines
at different wavelengths.
Finally we looked at the visible light emitted by molecular
nitrogen. In this case the spectrum was somewhere in between a
continous spectrum and a line spectrum. You were able to see
bands of colored light rather than clear line emitted by the nitrogen
(the bands are probably very closely spaced lines).
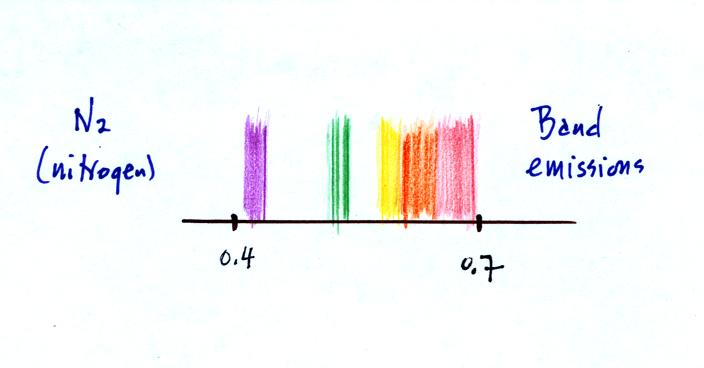
This is closer to being a continous spectrum but there were still
gaps, wavelengths without any emissions.
In the
same kind of way, air absorbs some wavelengths and transmits
others. We will need to worry about the filtering effect of the
atmosphere on ultraviolet, visible, and infrared light because all
three types of light are found in sunlight.
We will first look at the effect simple blue, green, and red glass
filters have on visible light. This figure wasn't shown in class.

If you try to shine white light (a mixture of all the colors) through a
blue filter, only the blue light passes through. The filter
absorption curve shows 100% absorption at all but a narrow range of
wavelengths that correspond to blue light. Similarly the green
and red filters only let through green and red light.
The following figure is a simplified easier to remember
representation of the
filtering effect of
the atmosphere on UV, VIS, and IR light (found on p. 69 in the
photocopied notes)
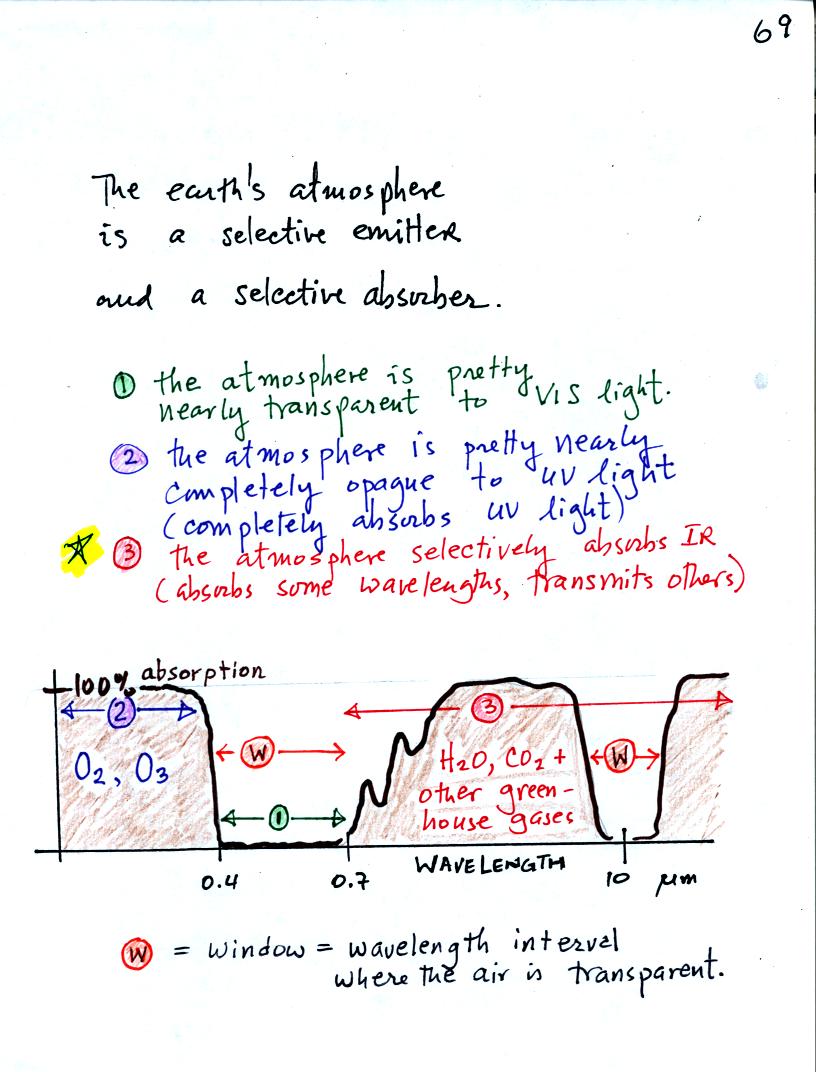
0% absorption means the
atmosphere behaves like a window
made of clear glass, the air is transparent to light. The light
can
pass freely through the atmosphere. 100% absorption on the other
hand
means the atmosphere is opaque to light, it blocks the light by
absorbing it.
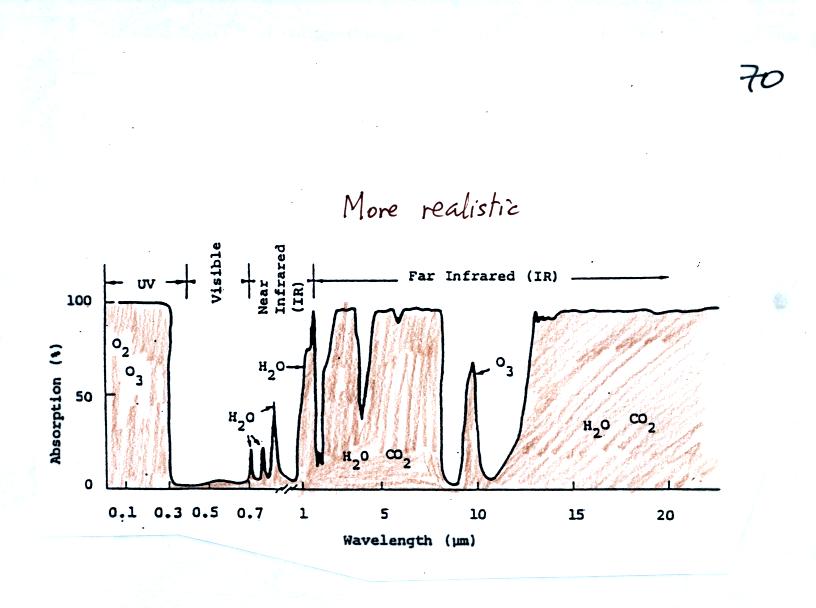
In our simplified representation oxygen and ozone make the atmosphere a
pretty good absorber of UV light The atmosphere is pretty nearly
perfectly
transparent to VIS light (we can check this out with our eyes, we can
see through the air, it is clear).
Greenhouse gases make the
atmosphere a
selective absorber of IR light - it absorbs certain IR wavelengths and
transmits others. It is the atmosphere's ability to absorb (and
also emit) certain wavelengths of infrared light that produces the
greenhouse effect and warms the surface of the earth.
Note "the atmospheric window"
centered at 10 micrometers. Light emitted by the earth at this
wavelength will pass through the atmosphere. Another transparent
region, another window, is found in the visible part of the spectrum.
The following figure found at the top of p. 70 in the photocopied Class
Notes is a more realistic filter absorption for the atmosphere.