Friday Oct. 19, 2007
All of the 1S1P Assignment #1 reports have now been graded.
The revised Expt. #1 reports have
also been graded.
Optional Assignment #5 (Humidity) is due at the beginning of class next
Monday.
1S1P Assignment #2 reports are due next Wednesday.
Experiment #3 reports are due Monday Oct.
29.
Good sunny weather is predicted for the next several days.
Collect your data soon so that you can return the experiment materials
next week and pick up the supplementary information sheet.
The revised Expt. #2 reports are also due Mon., Oct. 29.
Today and
Monday we will be discussing many of the phenomena below. They
all
involve cooling air to (and/or below) the dew point temperature.
The air becomes saturated (RH=100%) and water vapor begins to condense.
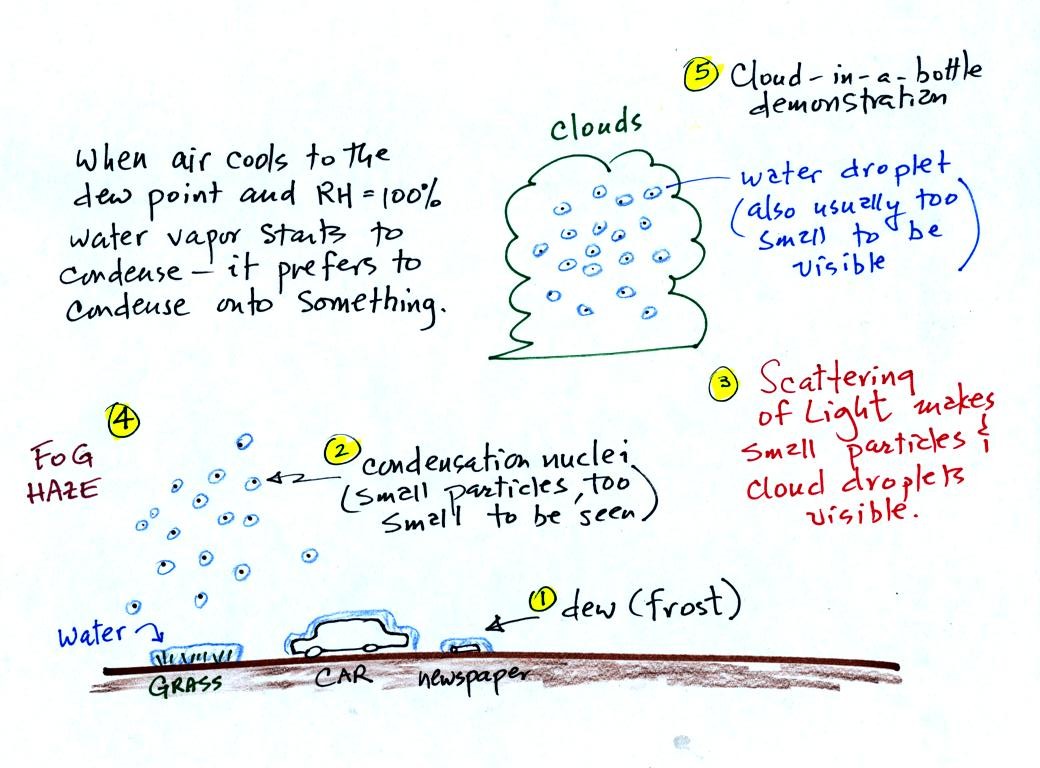
It turns out that it is much easier for water vapor to condense
onto
something rather than just forming a small droplet of pure
water Near the ground water vapor will condense onto cold
objects on the ground (the grass, automobile, and newspaper
above). In air above the ground water vapor condenses onto small
particles in the air called condensation nuclei. We'll learn a
little bit about these today.
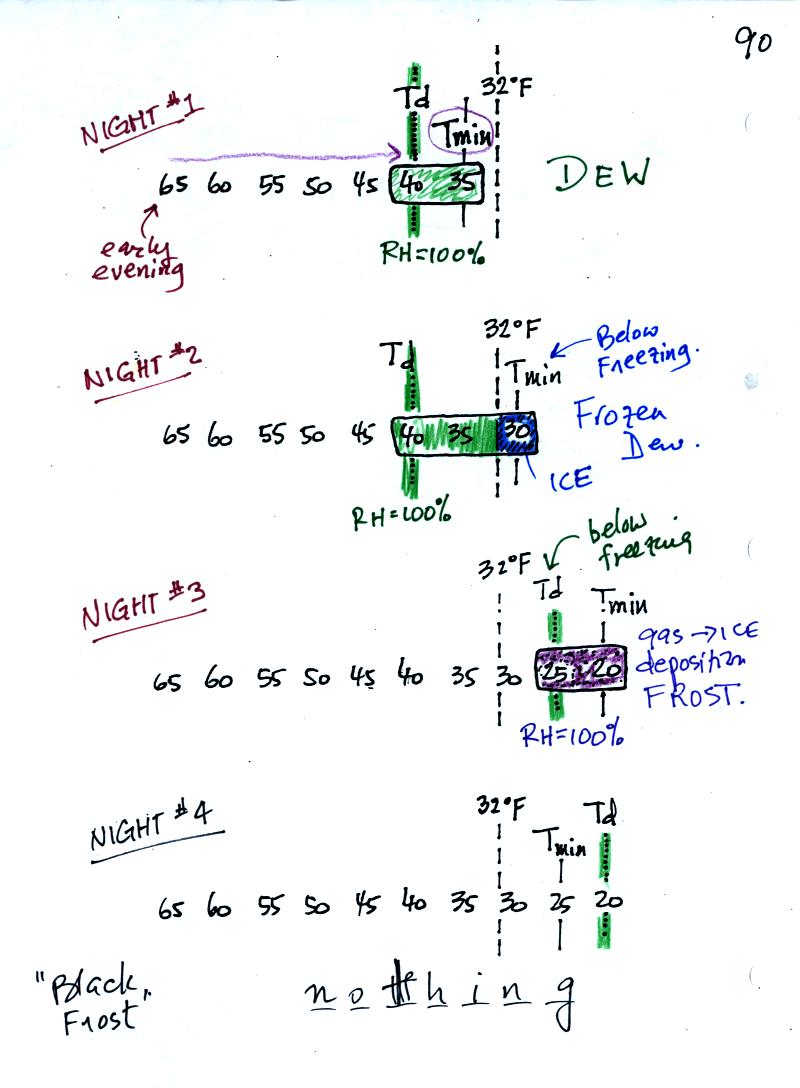
In the first example air starts out with a
temperature
of 65 F early in the evening. It cools to 35 F during the
night. When the air reaches 40 F, the
dew point, the RH reaches 100%. As the air temperature drops
below the dew point and cools to 35 F water vapor will condense onto
the ground or objects on the ground (such as an automobile). This
is dew.
The dew point is the same but the nighttime minimum temperature
is below freezing in the second example. Dew will form again on
this night when the
air temperature reaches 40 F. Once the air temperature drops
below 32 F though the dew will freeze and form frozen dew.
In the third example both the dew point and nighttime minimum
temperatures are below freezing. When the air temperature drops
below the dew point, water vapor turns directly to ice (deposition) and
forms frost.
The dew point in this case is sometimes called the frost point.
The air never becomes saturated in the fourth example because the
nighttime minimum temperature never cools to the dew point. You
wouldn't see anything on this night.
When air
above the ground reaches 100% relative humidity it is much easier for
water vapor to condense onto small particles in the air called
condensation nuclei than to just form a small droplet of water.
There are hundreds even thousands of these small particles in every
cubic centimeter of air. We can't see them because they are so
small.
You can learn why it is so hard to form small droplets of pure water by
reading the top of p. 92 in the
photocopied class notes.

Water vapor will condense onto certain kinds of condensation
nuclei
even when the relative humidity is below 100% (again you will find some
explanation of this on the bottom of p.
92). These are called hygroscopic
nuclei.
A short video showed how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of glass.
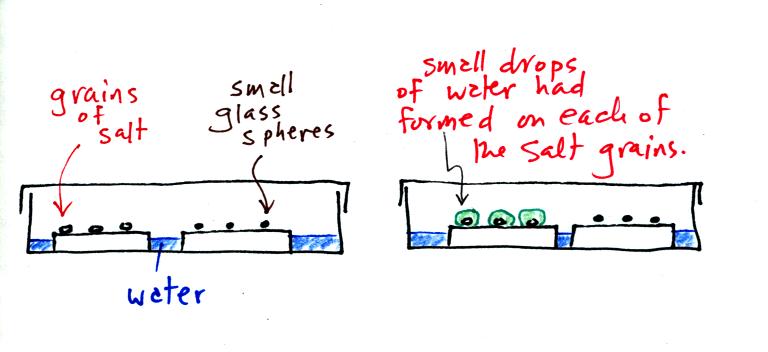
The start of the video at left showed the small grains of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right). The figure above wasn't shown in class.
In humid parts of the US, water will condense onto the grains of salt
in a salt shaker causing them to stick together. Grains of rice
apparently will keep this from happening and allow the salt to flow
freely out of the shaker when needed.
Even
though condensation nuclei and cloud droplets are too small to be seen,
we can tell when they are present because they scatter light. The
demonstration described below was done in class to make clearer what
scattering really is.
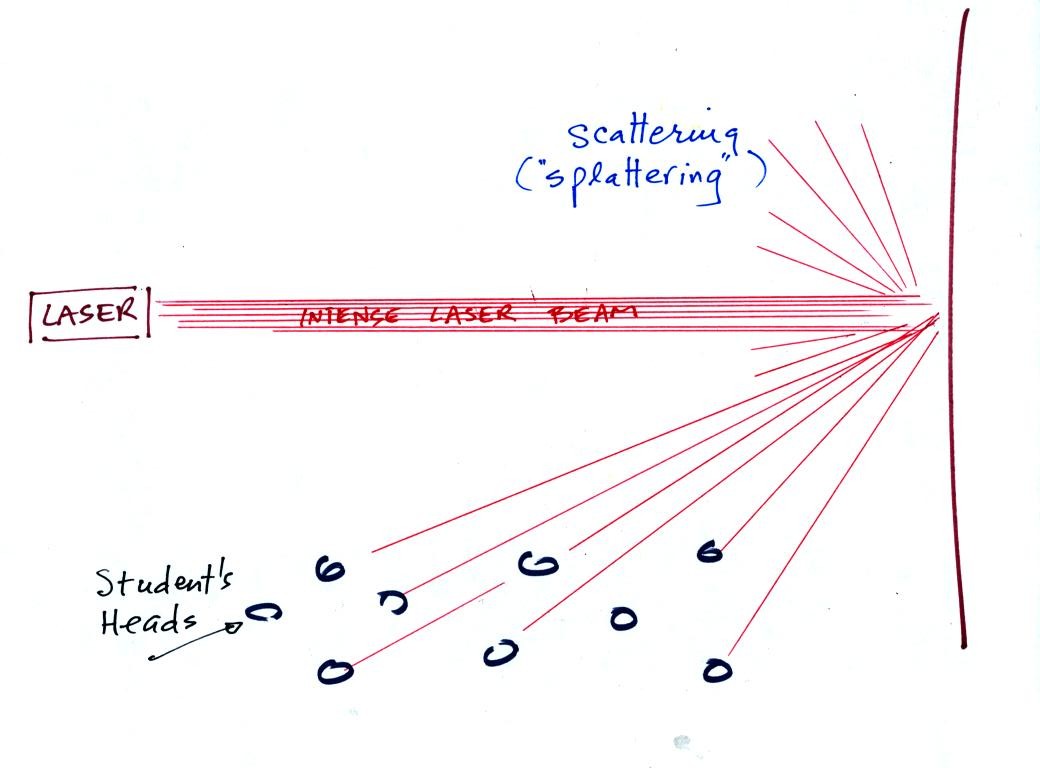
In the first part of the demonstration, a thin beam of bright red
laser light was shined across the
front of
the classroom. No one in the class could see this beam of
light. To see the beam you would need to stand over where the
beam struck the wall and look back toward the laser. The laser
light is very intense and could damage your eyes, so this wouldn't be a
very good thing to do.
Students in the class could see a red spot on the wall because the
light hitting the wall was scattered or splattered and sent off in a
multitude of directions. A individual ray of laser light was sent
to everyone
in the class (and because the intense light is split up into so many
rays,
the individual rays are weaker and safe to look at).
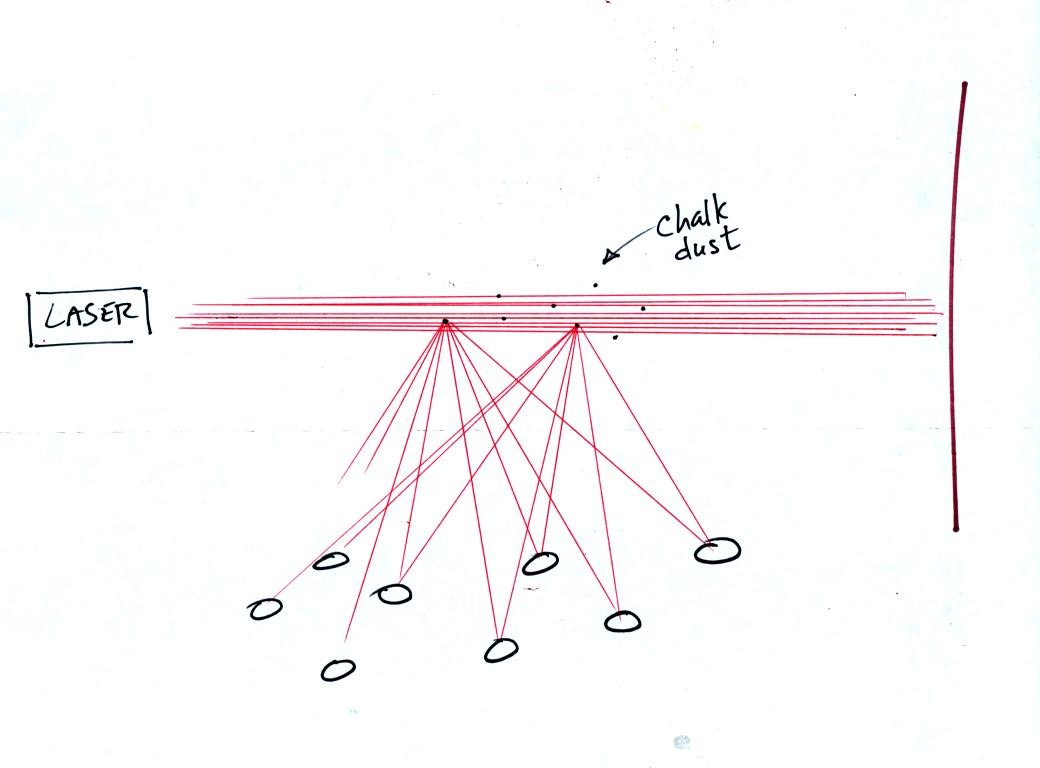
Next we clapped a couple of chalkboard erasers together. When
particles of chalk dust fell into the laser beam they intercepted some
of the laser light and scattered it. Again everyone in the room
got their own personal ray of light coming from each of the particles
of chalk. We use chalk because it is white, it scatters rather
than absorbs light. What would you have seen if black particles
of soot had been dropped into the laser beam?
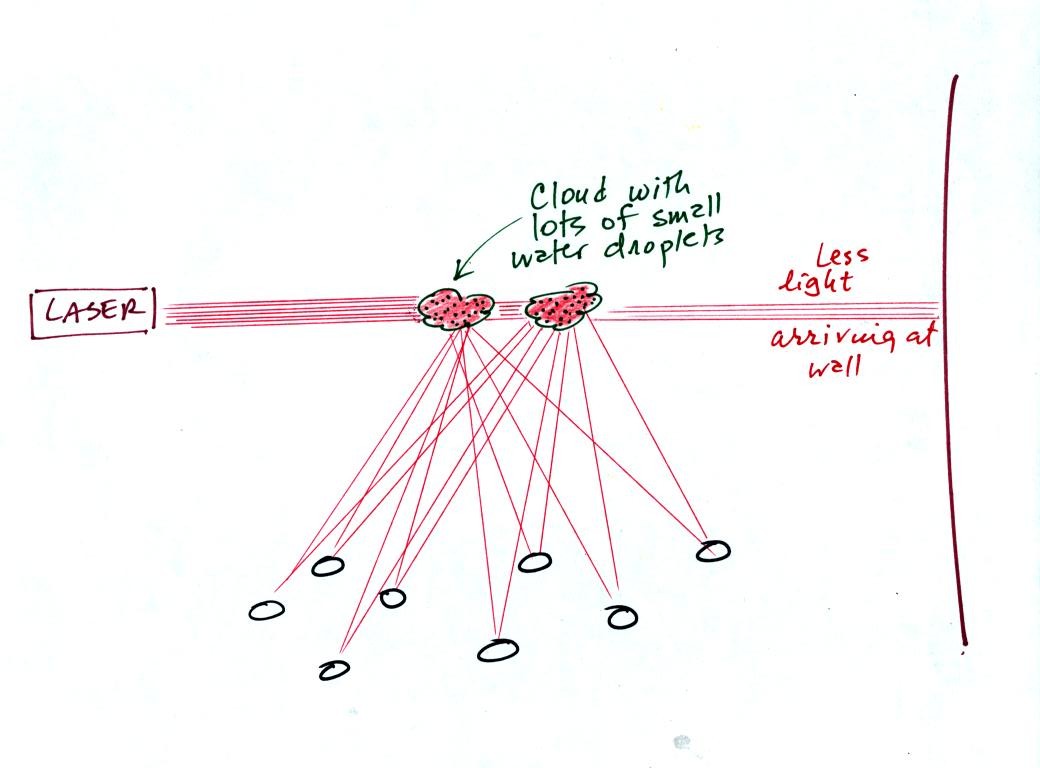
In the 3rd part of the demonstration we made a cloud by pouring some
liquid nitrogen into a cup of water. The numerous little water
droplets made very good scatterers. So much light was scattered
that the spot on the wall fluctuated in intensity (the spot dimmed when
lots of
light was being scattered, and brightened when not as much light was
scattered).
The blue color of the sky is caused by the scattering of light by air
molecules. The air molecules preferentially scatter the shorter
wavelengths in sunlight. We should at some point later in the
semester have time to discuss the blue color of the sky as well as
phenomena such as haloes and rainbows which involve the refraction
(bending) of light.
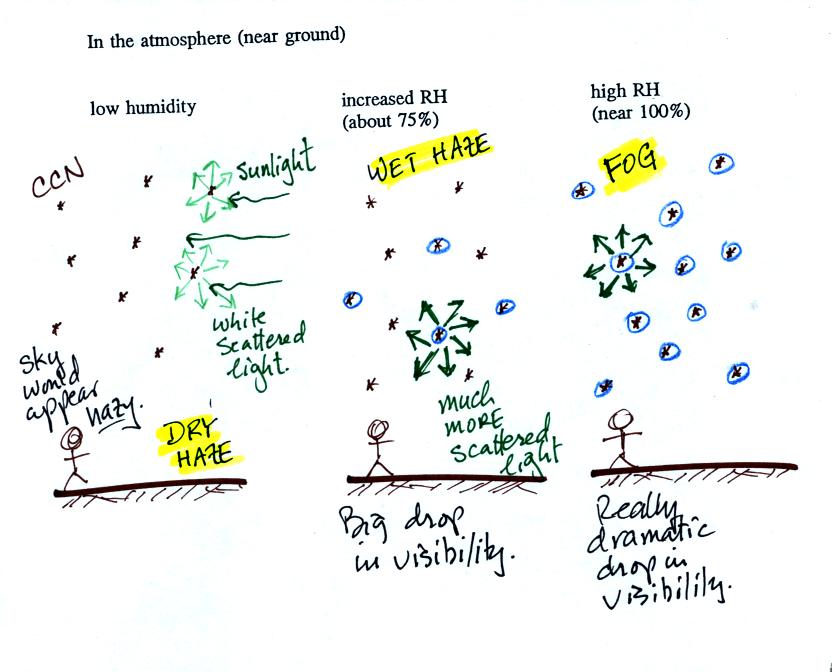
This figure (redrawn after class for improved clarity) shows how
cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter
sunlight. When you look at the sky you see the deep blue color
caused by scattering of sunlight by air molecules mixed together with
some white
light scattered by the condensation nuclei. This changes
the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze."
The middle picture shows what happens when you drive from the dry
southwestern part of the US into the humid
southeastern US. One of the first things you would notice is the
hazier
appearance of the air and a decrease in visibility. Because the
relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze."
Finally when the relative humidity increases to 100% fog forms.
Fog can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation nuclei. We
will see this effect in the cloud-in-a-bottle demonstration planned for
class next Monday.







