Monday Sept. 24, 2007
The graded Experiment #1 reports were returned in class today.
You are allowed to revise these reports. The revised reports are
due by Mon., Oct. 8. You only have to rewrite sections where you
want to earn additional credit. Please return the original report
with your revised report.
Some
material not covered in class last Friday (temperature vs heat,
temperature scales) was added to the Fri., Sep.
21 online notes. That material was discussed quickly at the
start of today's class.
Conduction is the first of four energy transport processes
that we
will cover. The figure below illustrates this process. A
hot object is stuck in the middle of some air.
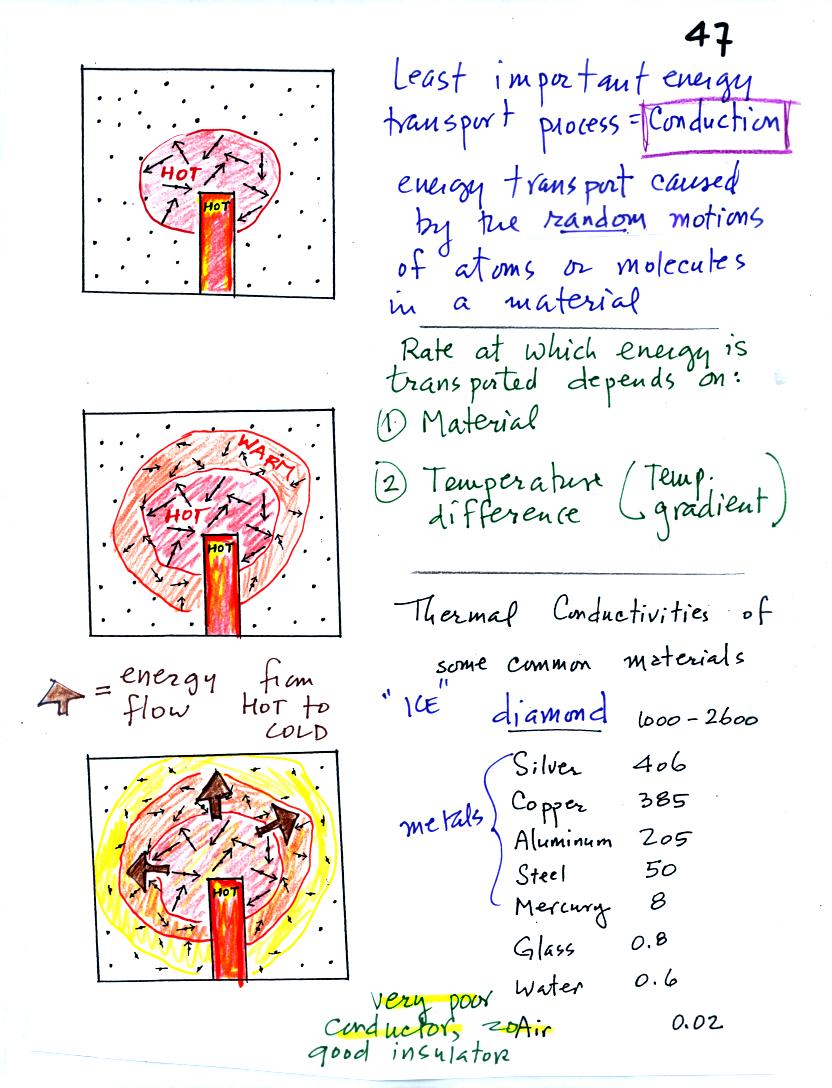
In the first picture the
random motions of the atoms or
molecules near the object have caused them to collide with and pick up
energy from the object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red). In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object. In
the third picture molecules further from the object now have gained
some energy (the yellow ones). The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
Conduction transports energy from hot to cold. The rate of
energy transport depends first on the material (air in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (sauce pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
The next figure shows a demonstration mentioned but not
performed in
class (the 3 figures weren't shown in class
either). It involves opening a bottle of something with a
strong
smell such as glacial acetic acid (acetic
acid gives vinegar its characteristic smell) in the front of the
classroom. With time the odor would eventually spread throughout
the class
room. This is an example of diffusion. The acetic acid
molecules would be moved through the room by random collisions with air
molecules. In many respects this is like the conduction of
heat. The demonstration wasn't performed because the
concentration of the acetic acid in the air, at least in the front of
the room, would be high enough to present a serious risk to the
instructor and students.
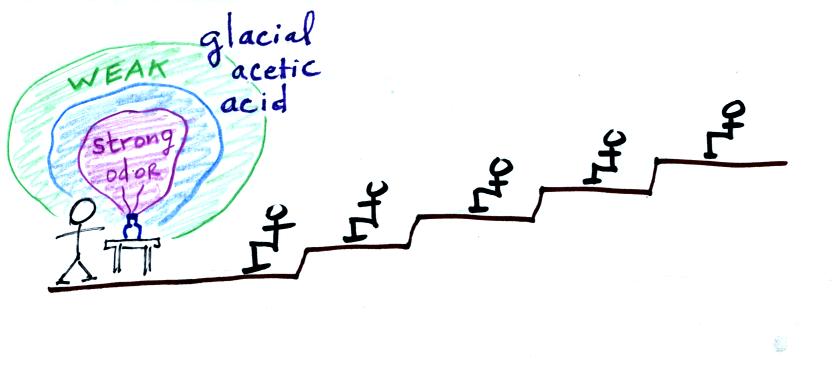
The acetic acid is beginning to evaporate into
the
air. Collisions with air molecules would begin to move the acetic
acid molecules toward the back of the room.
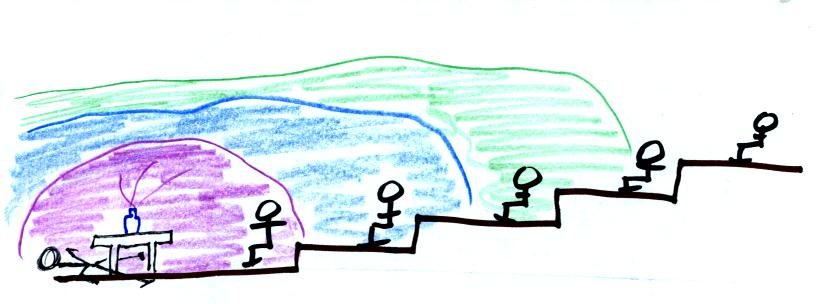
The instructor has lost
consciousness because of the
strong odor of the acetic acid in the front of the room.
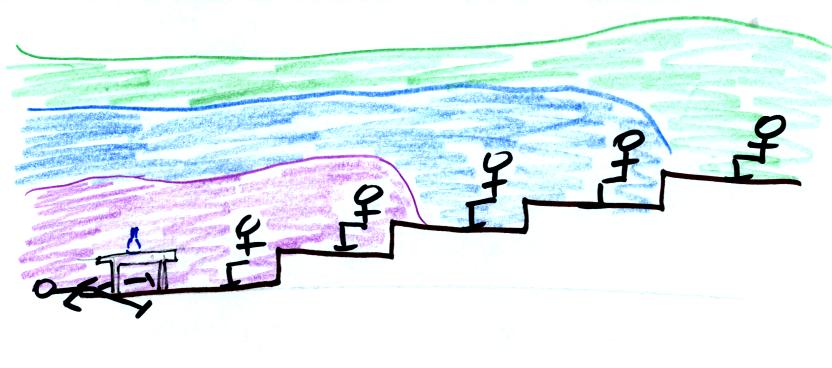
The odor would eventually spread
throughout the class
room.
Convection
was the next energy transport process we had a look at. Rather
than moving about randomly, the atoms or molecules move as a
group. Convection works in liquids and gases but not solids.
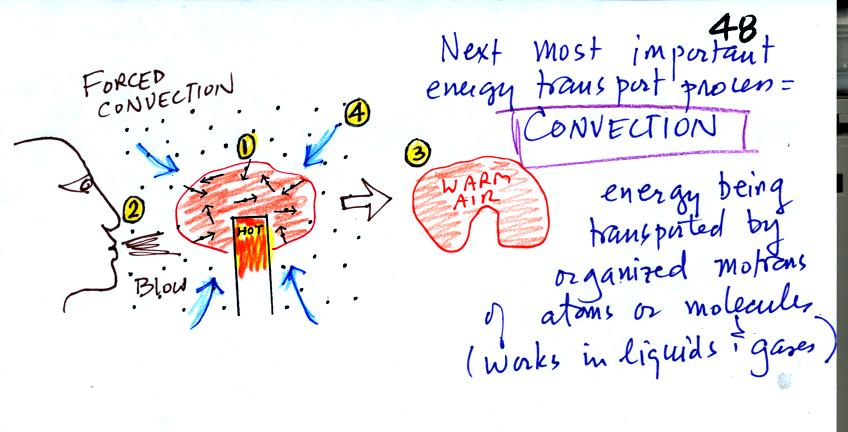
At Point 1 in the picture above a thin layer of air
surrounding a hot object has
been
heated by conduction. Then at Point 2 a person (yes that is a drawing
of a
person's head) is blowing the blob of warm air
off to the right. The warm air molecules are moving away at Point
3 from the
hot object together as a group (that's the organized part of the
motion). At Point 4 cooler air moves in and surrounds the hot
object and the cycle can repeat itself.
This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly.

A thin layer of air at Point 1 in the figure above (lower left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands as it
moves into lower pressure surroundings and cools. If the air is
moist, clouds can form). Cooler air moves in to take the place of
the rising air at Point 4 and the process repeats itself.
The example at upper right is also free convection. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.
Here's a little detour not taken in
class (we're beating this topic to death again).
Because air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection. Here are some examples of
insulators that use air:
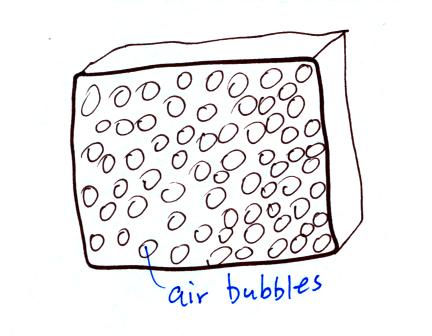
Foam contains lots of small bubbles of air

Double pane windows are used in many homes and buildings to conserve
energy. A thin layer of air is trapped between two panes of
glass. Window manufacturers also use a variety of other techniques
to make the windows even better insulators.
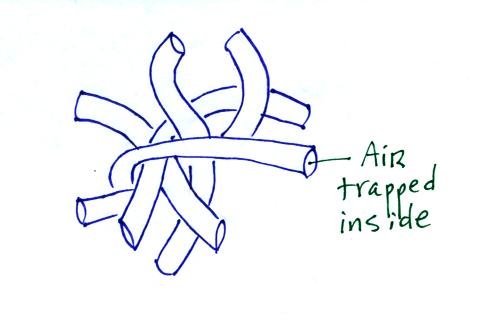
A tangled mess of hollow fibers (eg. hollofil) is often used in
sleeping bags or winter jackets.
Now some
practical applications of what we have learned about conductive and
convective energy transport.
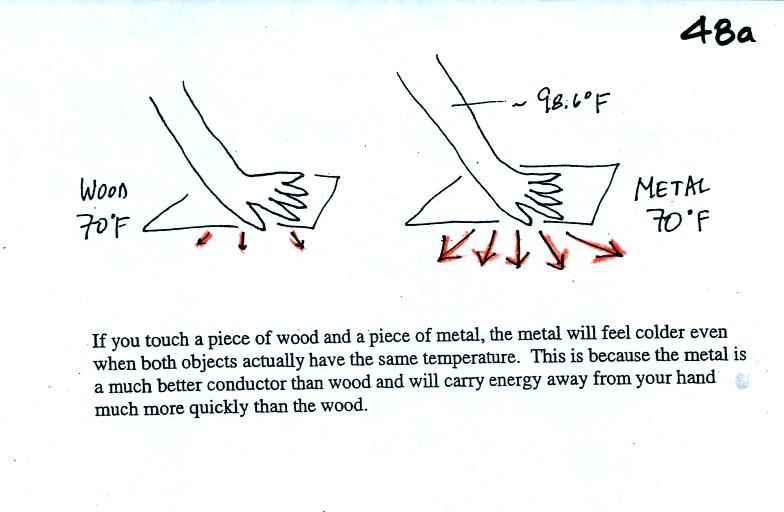
Metals are better conductors than wood. If you touch a
piece of
70 F metal it will feel colder than a piece of 70 F wood. A piece
of 70 F diamond would feel even colder because it is a better conductor
than metal. Something that feels cold may not be as
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
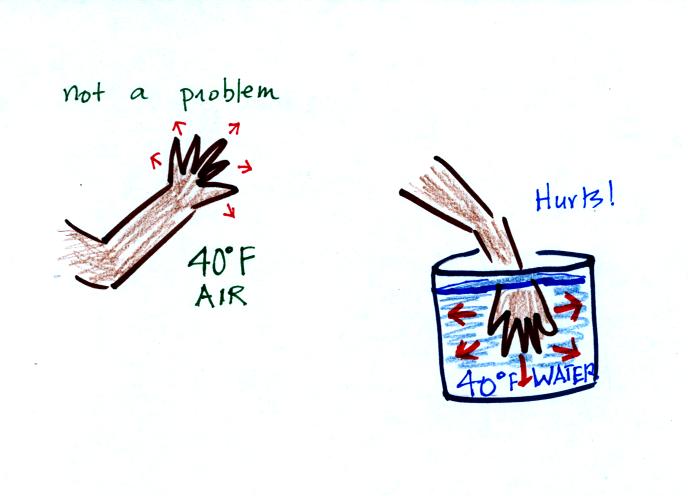
Air is a poor conductor. If you stick your hand out in 40 F
weather the air won't conduct energy away from your hand very quickly
at all and the air won't feel very cold. If you stick your hand
into a bucket of 40 F water, it will feel very cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water.

If you go outside on a 40 F day (calm winds) you will feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). A thermometer
behaves differently. It actually cools to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy.

If you go outside on a 40 F day with 30 MPH winds your body
will lose
energy at a more rapid rate (because convection together with
conduction are transporting energy away from your body). It will
feel colder than a 40
F day
with calm winds. Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a calm 28
F day. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
Standing outside on a 40 F day is usually not a life threatening
situation. Falling into 40 F water is.
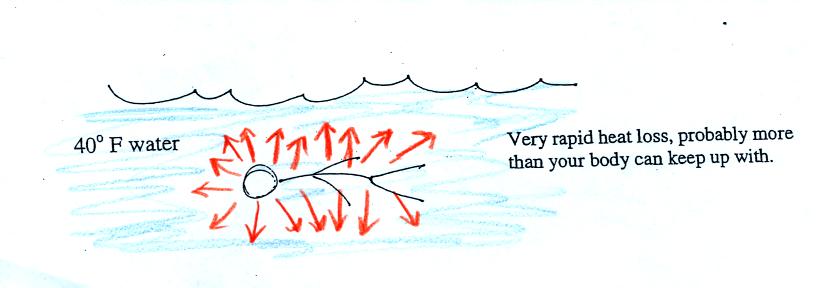
Energy will be conducted away from your body more quickly than your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia which can bring on
heatstroke and which is probably a more serious outdoors risk in S.
Arizona.













