Thursday Aug. 30, 2007
The Practice Quiz is one week from today. A
preliminary version
of the Practice Quiz Study Guide is now
available online (there probably won't be many changes made between now
and next week). Note the location of the reviews held before the
practice quiz aren't yet known.
The collection of old quizzes from a previous semester of this course
are now available for purchase ($2.50).
You should expect to see the first 1S1P Assignment and the first
Optional (Homework) Assignment soon.
Here is a
little more material concerning stratospheric ozone.
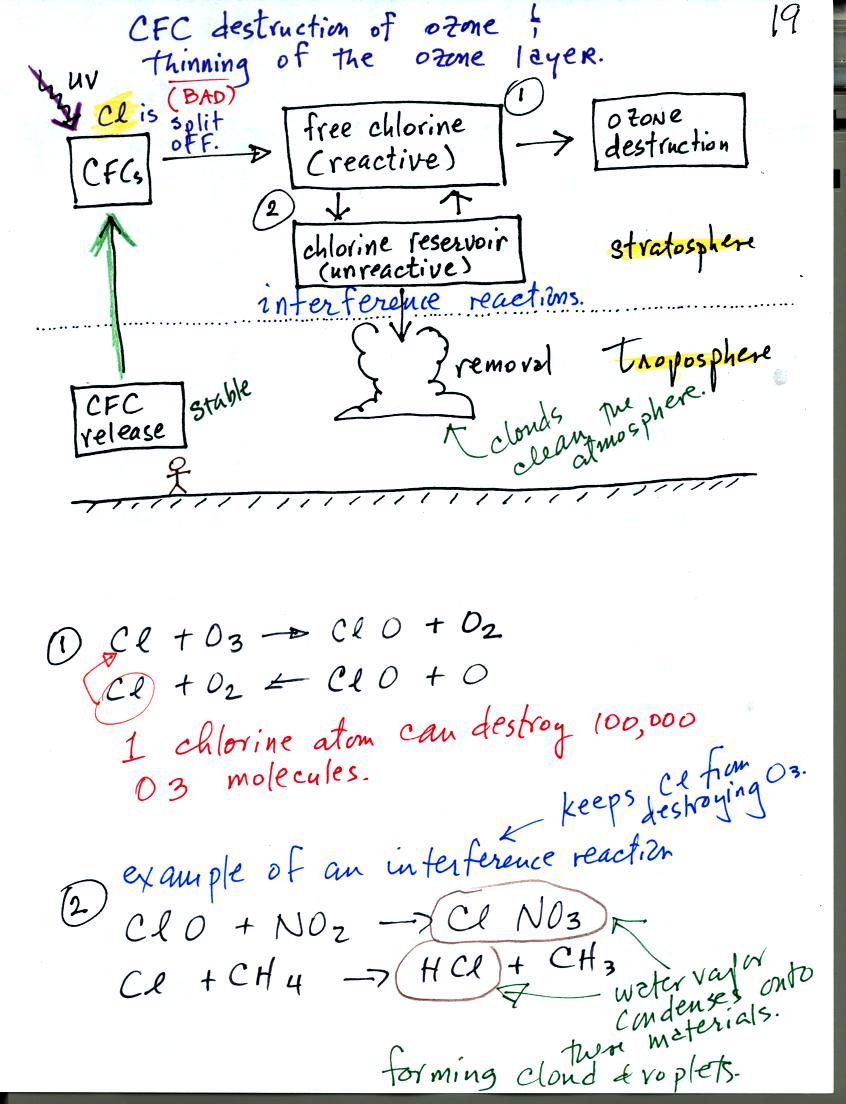
This figure illustrates destruction of stratospheric ozone
(thinning of the ozone layer) by chlorofluorocarbons (CFCs). CFCs
were at one time thought to be an ideal industrial chemical.
CFCs are unreactive, non toxic, and stable. Once they get into
the atmosphere they remain there a long time, as much as 100 years.
CFCs released at ground level remain in the atmosphere long enough that
they can eventually make
their way up into the stratophere. UV light can then break
chlorine
atoms off the CFC molecule. The resulting
"free chlorine" can react with and destroy ozone. This is shown
in (1) above. Note how the chlorine atoms reappears at the end of
the two step reaction. A single chlorine atom can destroy 100,000
ozone molecules.
There are ways of removing chlorine from the atmosphere. A couple
of these so called "interference reactions" are shown in (2)
above. The reaction products might serve as
condensation nuclei for cloud droplets (the small water drops that
clouds are composed of) or these gaseous products might dissolve in the
water in clouds. In either event the chlorine containing chemical
is removed from the atmosphere by falling precipitation. Clouds
are probably the most effective way of cleaning the atmosphere.
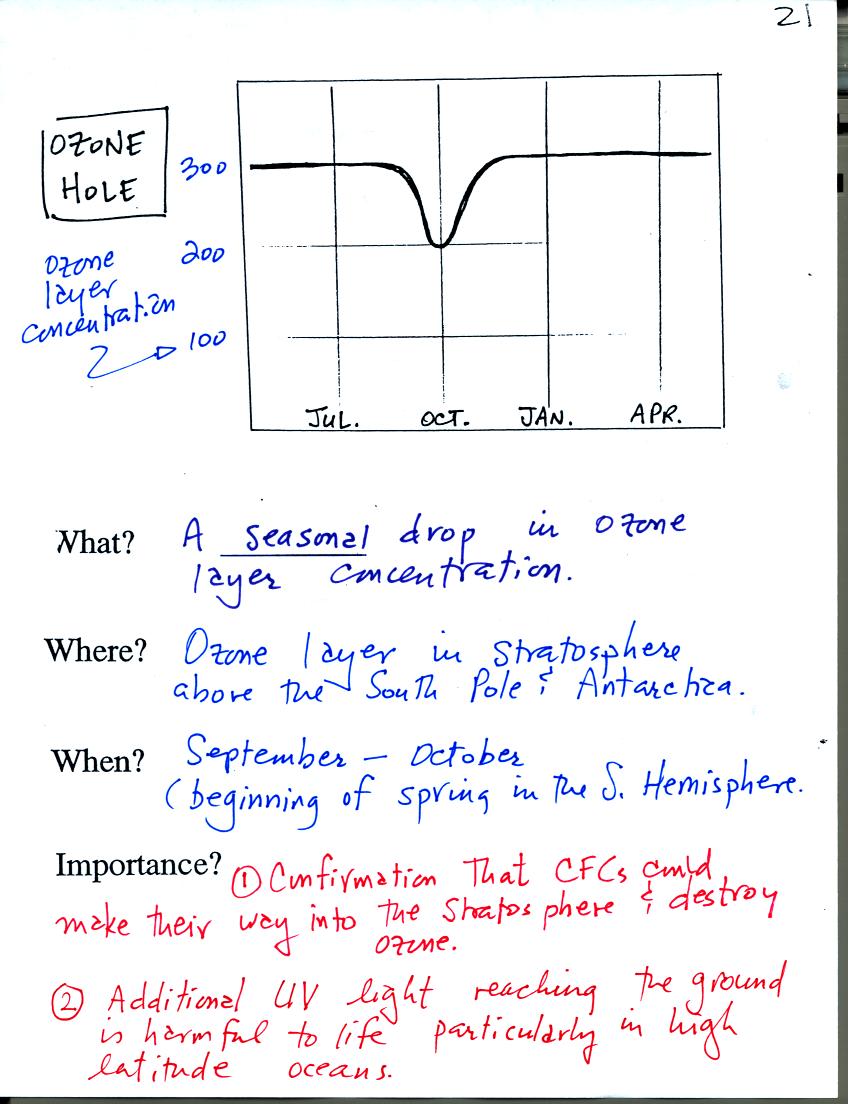
The ozone hole that forms above the S. Pole every year
around October
was one of the first real indications that CFCs could react with
and destroy stratospheric ozone. The hole is not really a hole in
the ozone layer, rather the ozone layer thins (concentration drops)
significantly.

The discussion above explains how extremely cold
temperatures and an
unusual wind pattern above the S. Pole in the winter are thought to
create the ozone hole when the sun returns in the spring.
Basically a new series of reactions (that take place on the surface of
cloud particles) interfere with the interference reactions. The
interference reactions would ordinarily keep chlorine from reacting
with and
destroying ozone. Interfering with those interference reactions
makes the chlorine available again to react with and destroy
ozone.
Chlorine containing compounds build up during the winter and are able
to destroy ozone once the sun returns in the spring.
Now we
really need to move into the middle portion of Chapter 1. Before
doing that we will learn go back to sulfur dioxide and learn a little
bit about acid rain.

Some of what we learned about sulfur dioxide in class last Tuesday is
listed above. Some more information about acid rain (from p. 12
in the photocopied Class Notes) is given below.
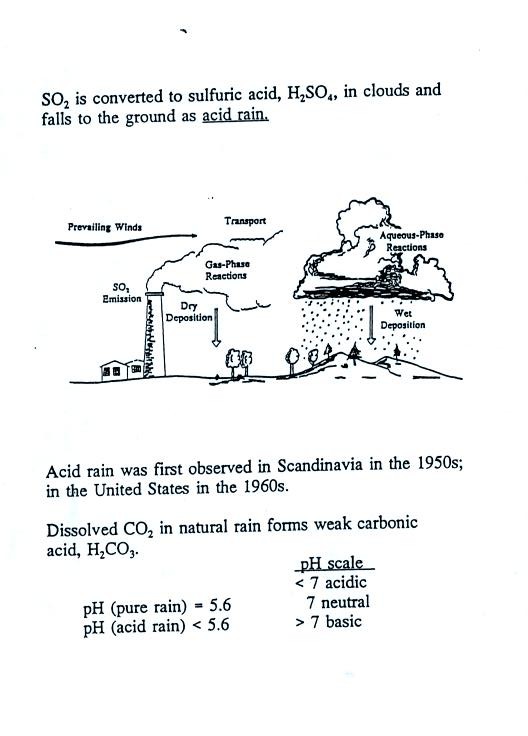
Note that clean unpolluted rain has a pH less than 7 and is slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. Acid rain is often a problem in regions that are
100s even 1000s of miles from the source of that sulfur dioxide that
forms the acid rain.

Some of the problems or consequences of acid rain.
A short colorful acid rain
demonstration was done in class. Carbon dioxide gas was used
instead of sulfur dioxide.
Now we're
finally ready to start the middle
portion of Chapter 1 and look at how atmospheric characteristics such
as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we will spend some time
trying to understand what pressure is and what can cause it to change.
We will start by looking at how air temperature changes with altitude
because that is a property that were are able to feel and are probably
most familiar with.
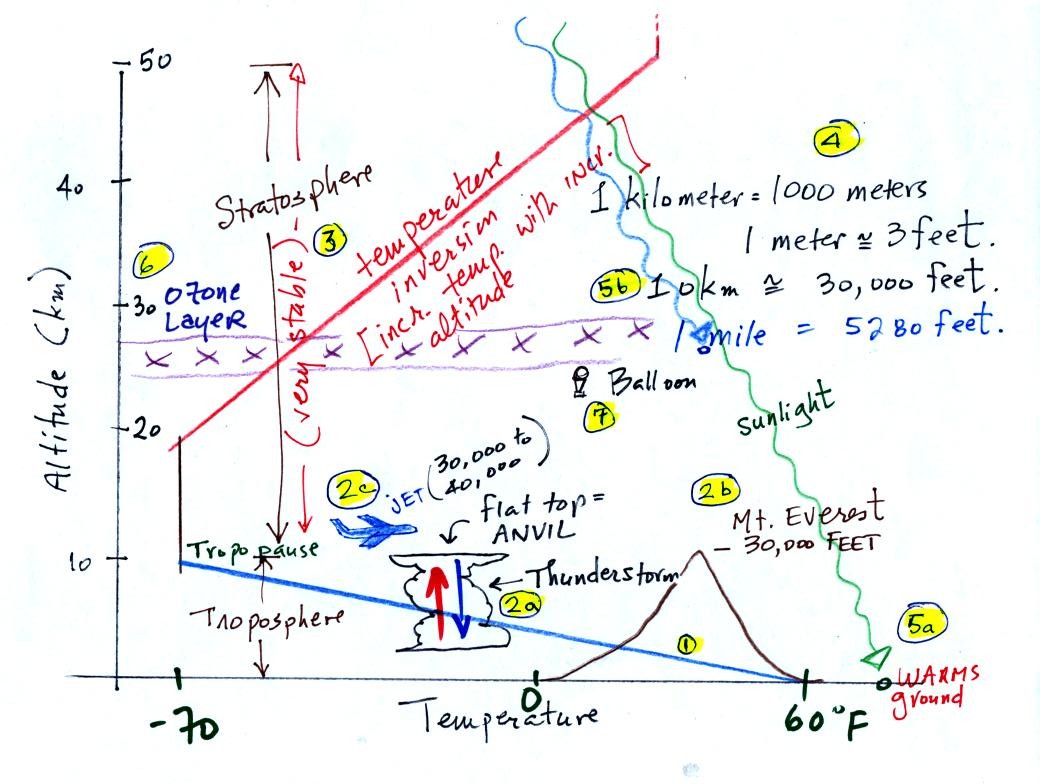
There's a lot of information on this figure. We'll work through
it number by number (the numbers
were added after class). With a little work you should be
able to start with a blank sheet of paper and recreate this figure on
your own.
The atmosphere can be split
into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. There
are additional layers (the mesosphere and thermosphere) above 50 km but
we won't worry about them.
1. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature usually decreases
with
increasing altitude.
The troposphere contains most of the water vapor
in the atmosphere and is where most of the weather occurs. The
troposphere can be stable or unstable (tropo means to turn over and
refers to the fact that air can move up and down in the
troposphere).
2a. The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are occurring. When the thunderstorm reaches the
top of the troposphere, it runs into the stable stratosphere. The
air can't continue to rise into the stable stratosphere so the cloud
flattens out and forms an anvil (anvil is the name given to the flat
top of the thunderstorm). The flat anvil top is something
that you can see and often marks the top of the troposphere.
2b. The summit of Mt. Everest is nearly 30.000 ft. tall and is
close to the top of the troposphere.
2c. Cruising altitude in a passenger jet is usually between
30,000 and 40,000, near or just above the top of the troposphere.
3. Temperature remains constant between 10 and 20
km
and then
increases with increasing altitude between 20 and 50 km. These
two sections form the stratosphere. The stratosphere is a
very stable air layer. Increasing temperature with increasing
altitude is called an
inversion. This is what makes the stratosphere so stable.
4. 10 km (kilometers) is approximately 30,000 feet or
about 6 miles.
5a. Much of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air just above. As you get further and
further from the warm ground, the air is colder and colder. This
explains why air temperature decreases with increasing altitude.
5b. How do you explain increasing temperature with
increasing
altitude in the stratosphere.
Absorption of
ultraviolet light by ozone warms the air in the stratosphere and
explains why the air can warm. The air in the stratosphere is
much less dense (thinner) than in the troposphere. It doesn't
take as much energy to warm this thin air as it would to warm denser
air closer to the ground.
6. The ozone layer is found in the
stratosphere (peak concentrations are found near 25 km altitude).
7. That is a manned balloon.
Auguste Piccard and Paul Kipfer were the first men to travel into the
stratosphere; (see pps 31 & 32 in
the photocopied Class Notes). We'll see a short video
showing part of their adventure in the next week or two.
What
follows is a little more detailed
discussion of the basic concepts of mass, weight, and density than was done in class.
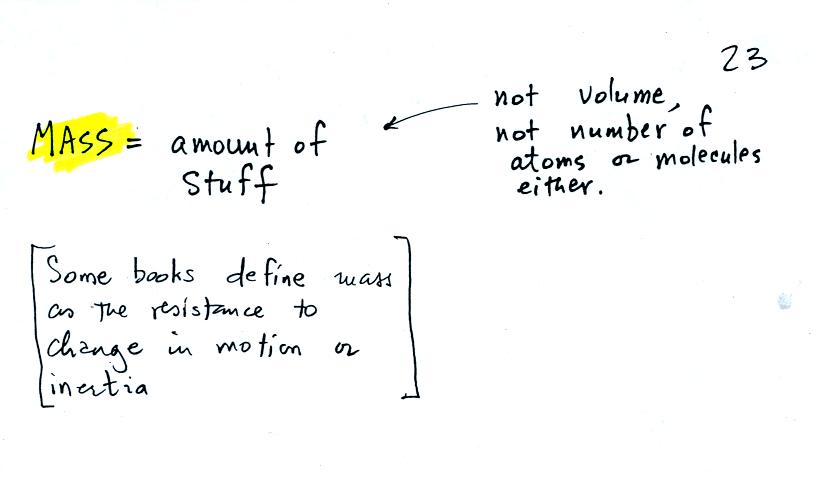
Before we can learn about atmospheric pressure, we
need to review
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
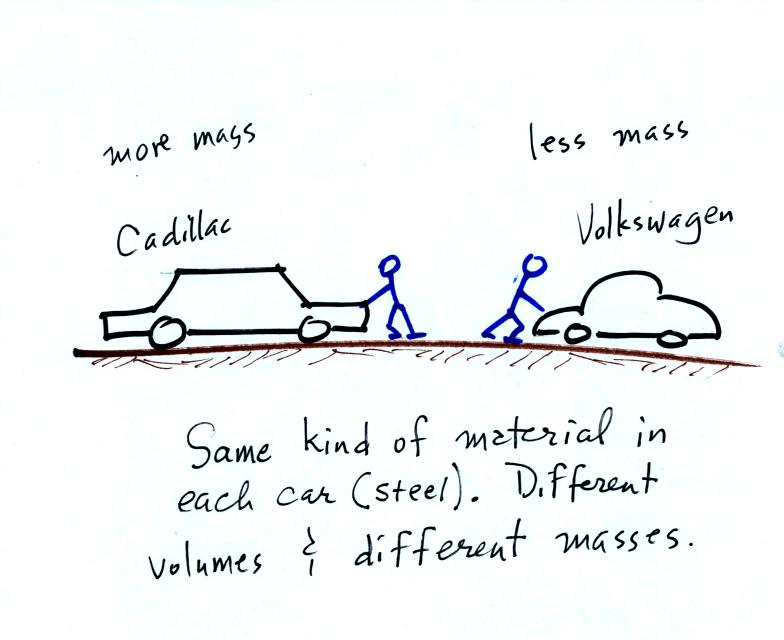
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down if it were
already moving).
It is possible to have two objects with the same
volume but very
different masses. Here's an example:
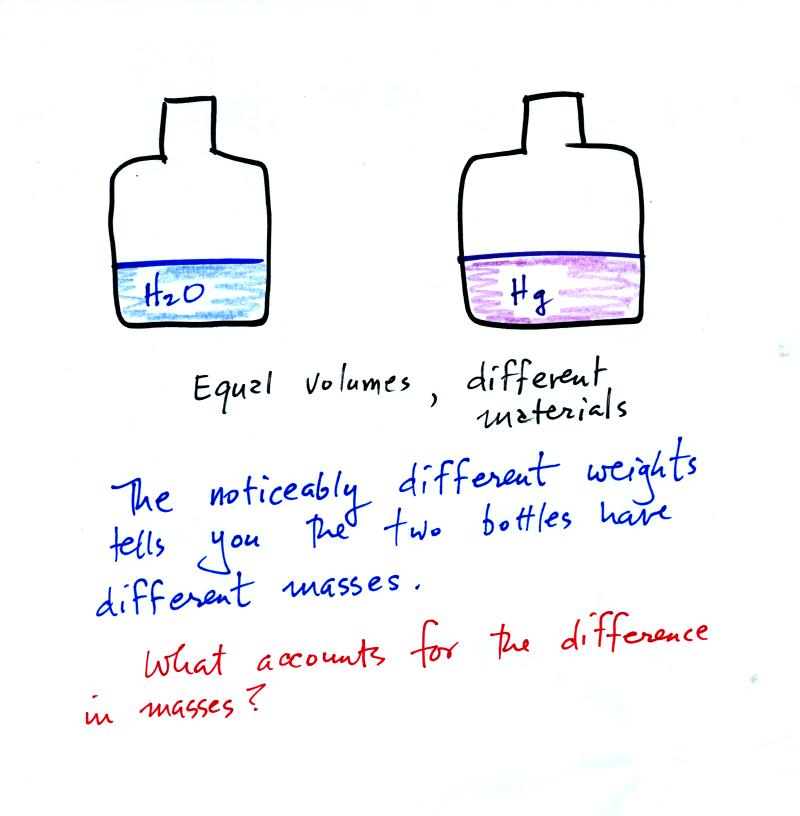
Bottles containing equal volumes
of water and mercury were
passed around in class (thanks for being careful with the bottles of
mercury). The bottle of mercury was quite a bit heavier than the
bottle of water.
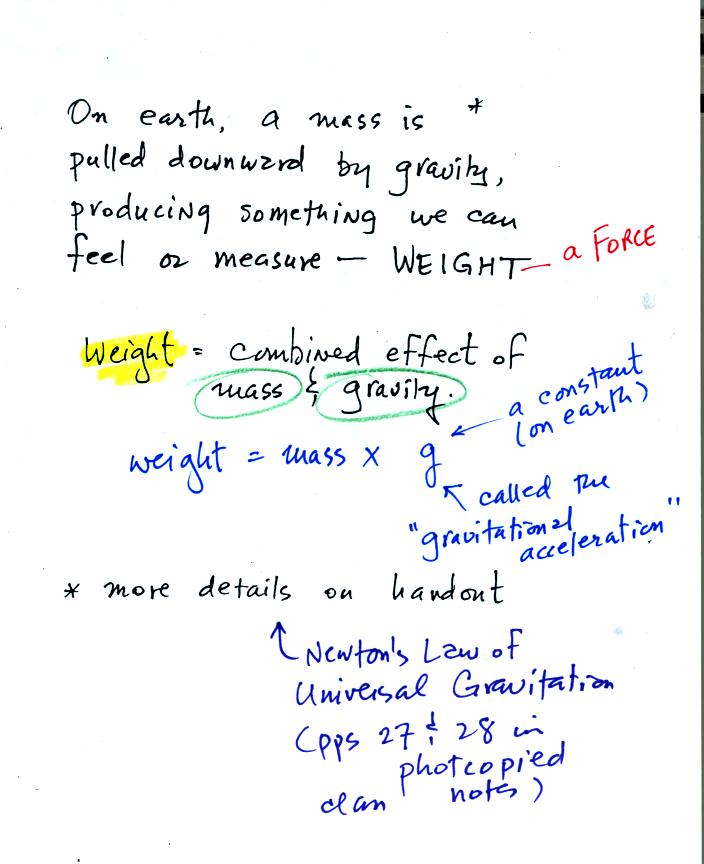
Weight is a force and depends on
both the mass of an object and the
strength of gravity.
We tend to use weight and mass interchangeably
because we spend all our
lives on earth where gravity never changes.
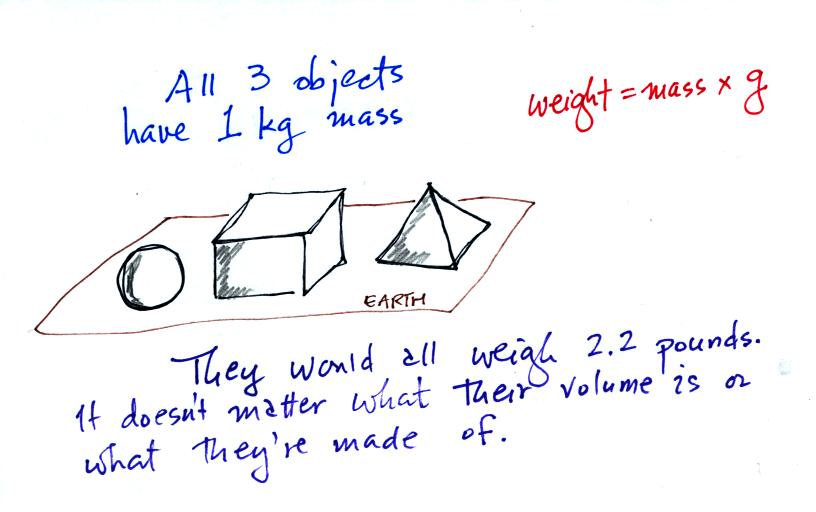
Any three objects that all have the same mass
would
necessarily have the same weight. Conversely
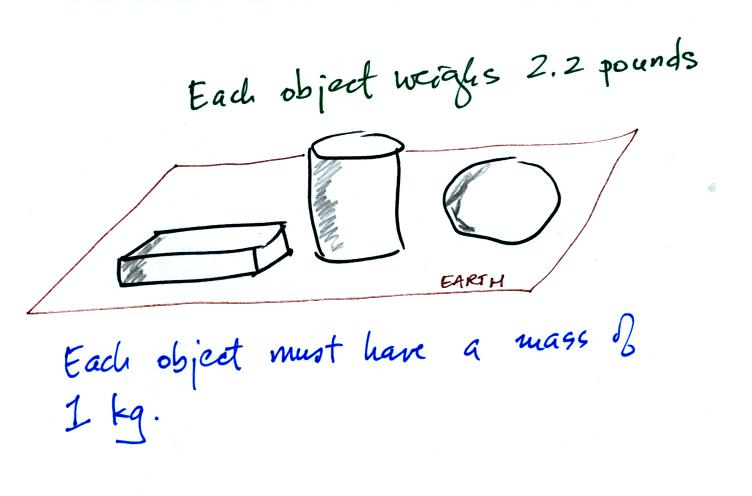
Three objects with the same weight
would have the same mass.
The difference between mass and weight is clearer (perhaps) if you
compare the situation on the earth and on the moon.
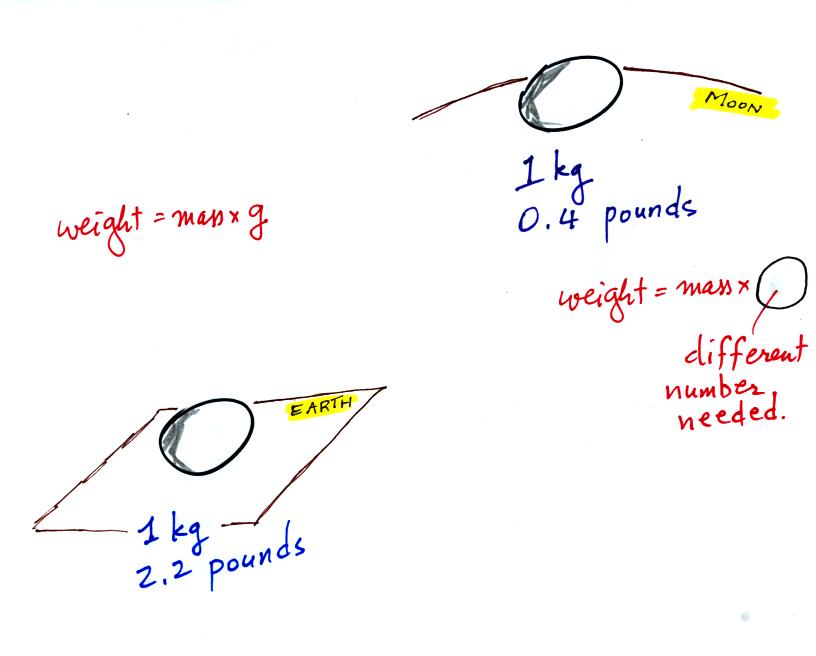
If you carry an object from the
earth to the moon, the mass
remains the
same (its the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.
Now
back to the bottles of water and mercury. The different weights
told us there were different masses. The volumes were equal, how
can we account for the differences in mass?
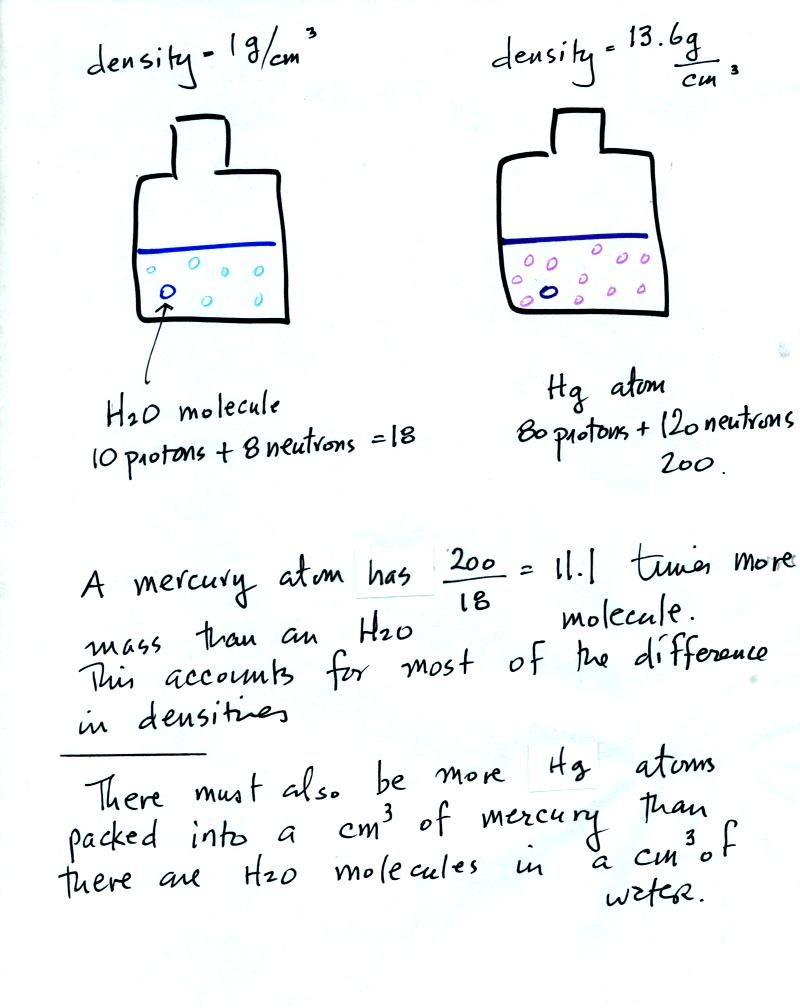
Mercury atoms are built up of many
more protons and neutrons
than a water molecule (also more electrons but they don't have nearly
as much mass as protons and neutrons). The mercury atoms have
11.1 times as much mass as the water molecule. This doesn't quite
account for the 13.6 difference in density. Despite the fact that
they contain more protons and neutrons, the mercury atoms must also be
packed closer together than the molecules in water.
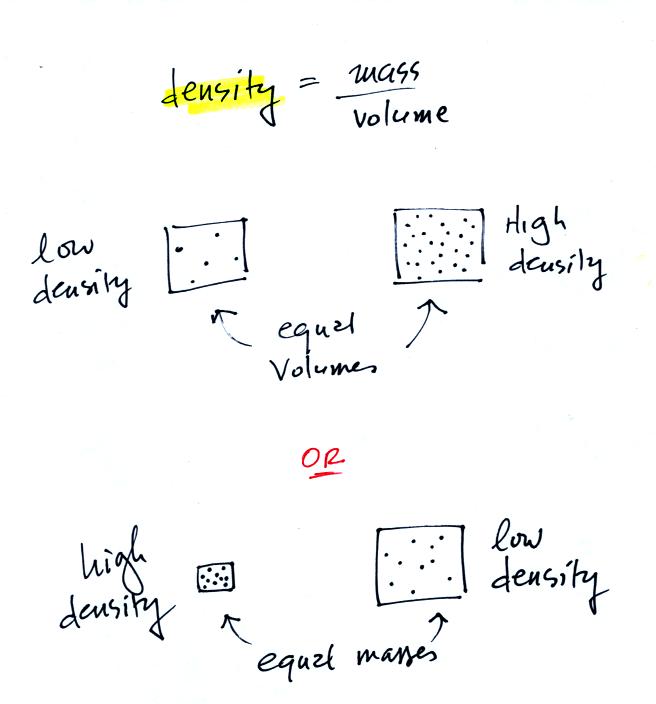
Definition and illustrations of
high and low density.















