Thursday Oct. 18, 2007
Optional Assignments #4 (Controls of Temperature) and #5 (Humidity) are
both due at the beginning of class next Tuesday. The in-class
Optional Assignment was returned in class today (the answers were added
to the Tue., Oct. 16 notes)
1S1P Assignment #2 reports are due one week from today (Thu., Oct.
25). All of the Assignment #1 reports have now been graded.
We need to
work several humidity problems. By doing these problems you
should become more
familiar with the humidity variables (mixing ratio, saturation mixing
ratio, relative humidity, and dew point temperature). You'll also
learn "how they behave" and what can cause each of these variables to
change value.
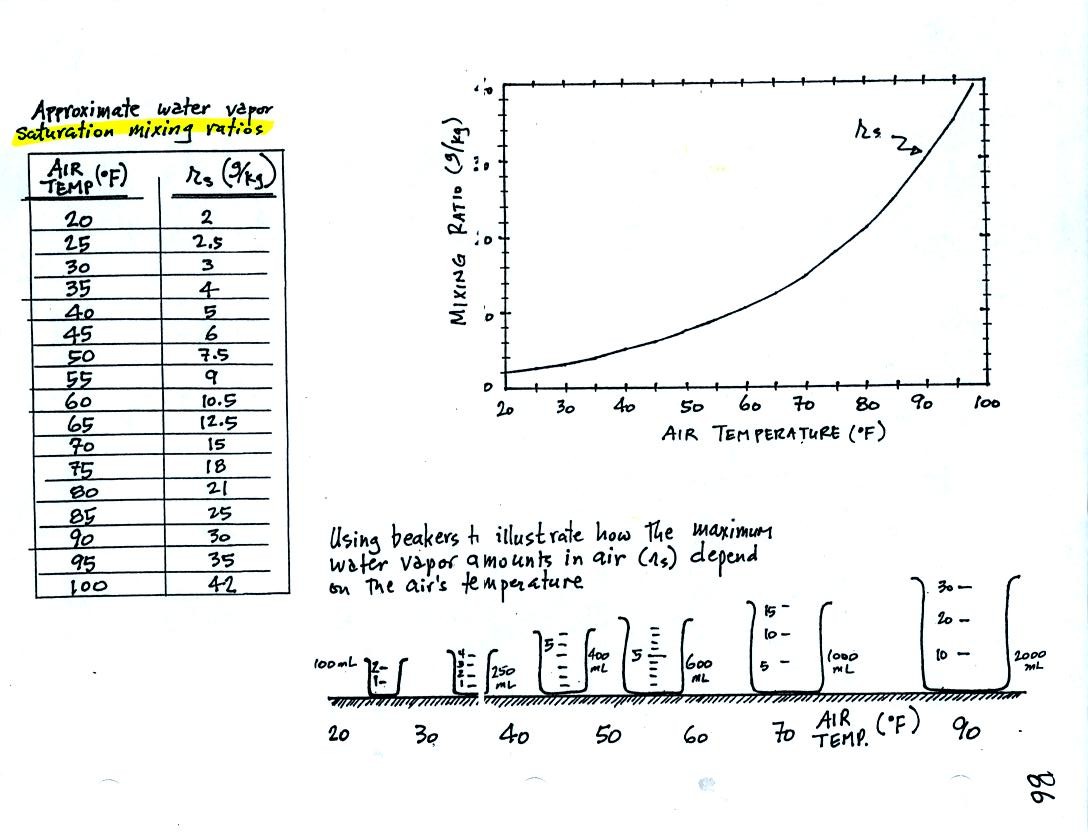
Keep this compilation of saturation mixing ratio values
(shown in a table and on a graph) handy, we will
use it a lot as we work through the humidity problem examples.
Remember that saturation mixing ratio is the maximum amount of water
vapor that can be found in air. It is a property of the air and
depends on the air's temperature.
The beakers (beakers were also brought to class)
are meant to show graphically the relative amounts of water
vapor that air at different temperatures can contain.
Now
the first of 4 example problems (actually done in class on Tuesday,
Oct. 16)

Here is what we actually did in
class.
You might have a hard time unscrambling this if you're seeing it for
the first
time. The series of steps that we followed are retraced
below:

We're given an air temperature of 90 F and a mixing ratio
(r) of 6
g/kg. We're supposed to find the relative humidity (RH) and
the dew point temperature.
We start by entering the data we were given in the
table. Once
you know the air's temperature you can look up the saturation mixing
ratio value; it is 30 g/kg for 90 F air. 90 F air could
potentially hold 30 grams of water vapor per kilogram of dry air (it
actually contains 6 grams per kilogram in this example).
Once you know mixing ratio and saturation mixing ratio you can
calculate the relative humidity. The RH is 20%.

The numbers we just figured out are shown on the top line
above.
(A) We imagined cooling the air from 90F to 70F, then to 55F, and
finally to 45F.
(B) At each step we looked up the saturation mixing ratio and entered
it on the chart. Note that the saturation mixing ratio values
decrease as the air is cooling.
(C) The mixing ratio doesn't change as we cool the air. The only
thing that changes r is adding or removing water vapor and we aren't
doing either.
(D) Note how the relative humidity is increasing as we cool
the
air. The air still contains the same amount of water vapor it is
just that the air's capacity is decreasing.
Finally at 45 F the RH becomes 100%. The dew point temperature in
this problem is 45 F.
What would happen if we cooled the air further still, below the dew
point temperature?
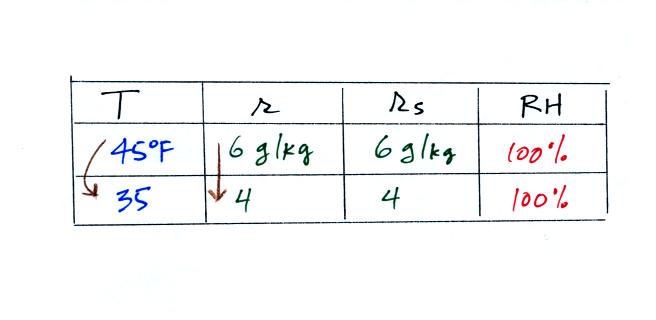
35 F air can't hold the 6 grams of water vapor
that 45 F air can. You can only "fit" 4 grams of water vapor into
the 35 F air. The remaining 2 grams would condense. If
this happened at ground level the ground would get wet with dew.
If it happens above the ground, the water vapor condenses onto small
particles in the air and forms fog or a cloud. Now because water
vapor is being taken out of the air (and being turned into water), the
mixing
ratio will decrease from 6 to 4. That is why the mixing ratio is
changing.
In many ways cooling moist air is liking squeezing a
moist sponge (the figure below
wasn't shown in class)

Squeezing the
sponge and reducing its volume is like cooling moist air and reducing
the saturation mixing ratio. At first when you sqeeze the sponge
nothing happens, no water drips out. Eventually you get to a
point where the sponge is saturated. This is like reaching the
dew point. If you squeeze the sponge any further (or cool air
below
the dew point) water will begin to drip out of the sponge (water vapor
will condense from the air).
Now
Problem #2 (this is where we actually started in class on Thursday)
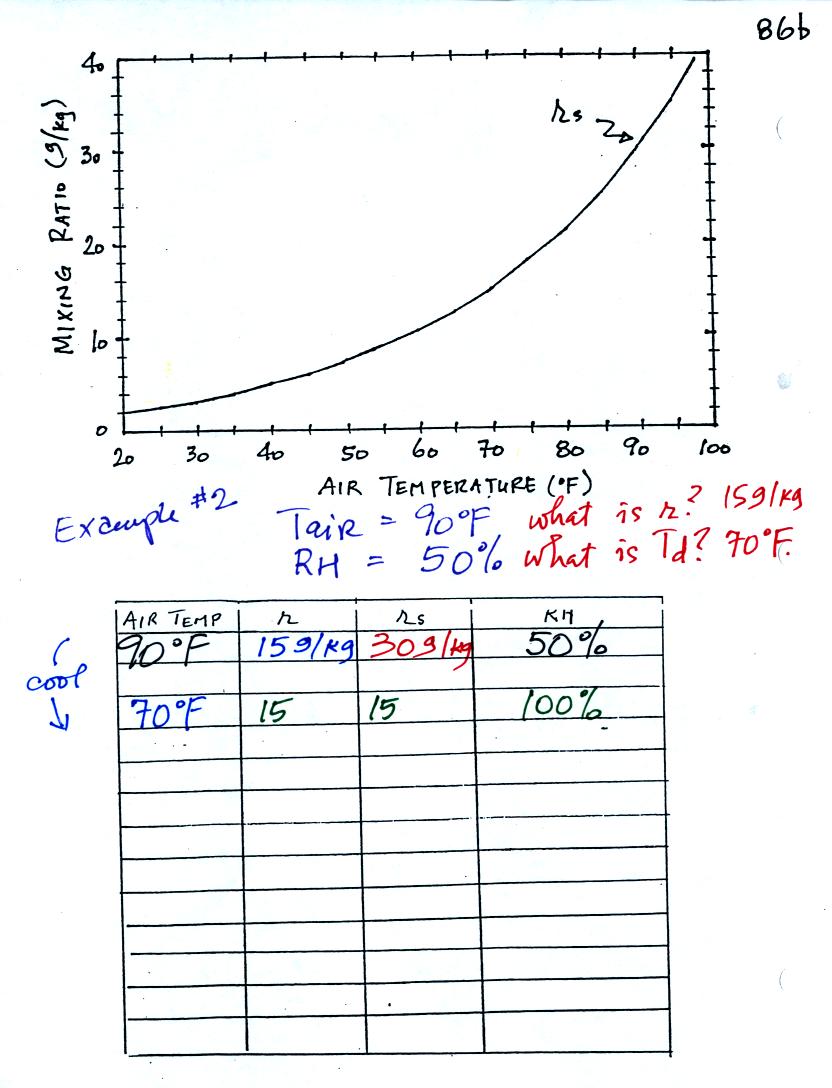
Here is what we did in class. Given an air
temperature
of 90
F and a relative humidity of 50% you are supposed to figure out the
mixing ratio (15 g/kg) and the dew point temperature (70 F). The
problem is worked out in detail below:
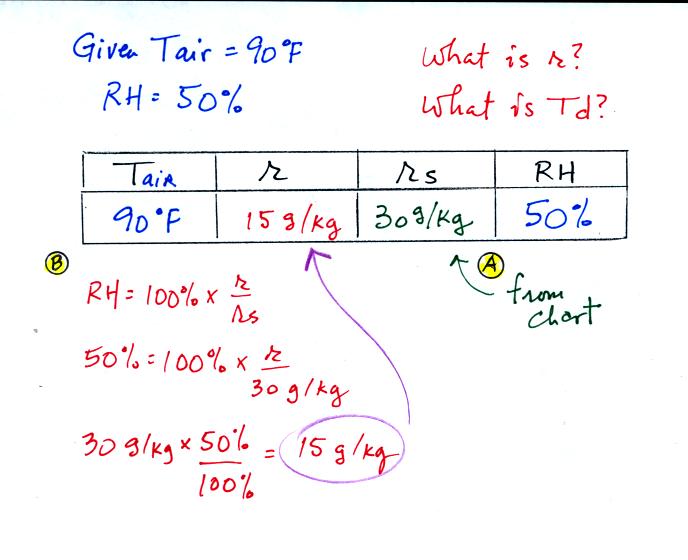
First you fill in the air temperature and the RH data that
you are
given.
(A) since you know the air's temperature you can look up the
saturation mixing ratio (30 g/kg).
(B) Then you can substitute into
the relative humidity formula and solve for the mixing ratio (15 g/kg).

Finally you imagine cooling the air. Cooling causes
the
saturation mixing ratio to decrease, the mixing ratio stays constant,
and the relative humidity increases. In this example the RH
reached 100% when the air had cooled to 70 F. That is the dew
point temperature.
We can use
results from humidity problems #1 and #2 to
learn a useful rule.
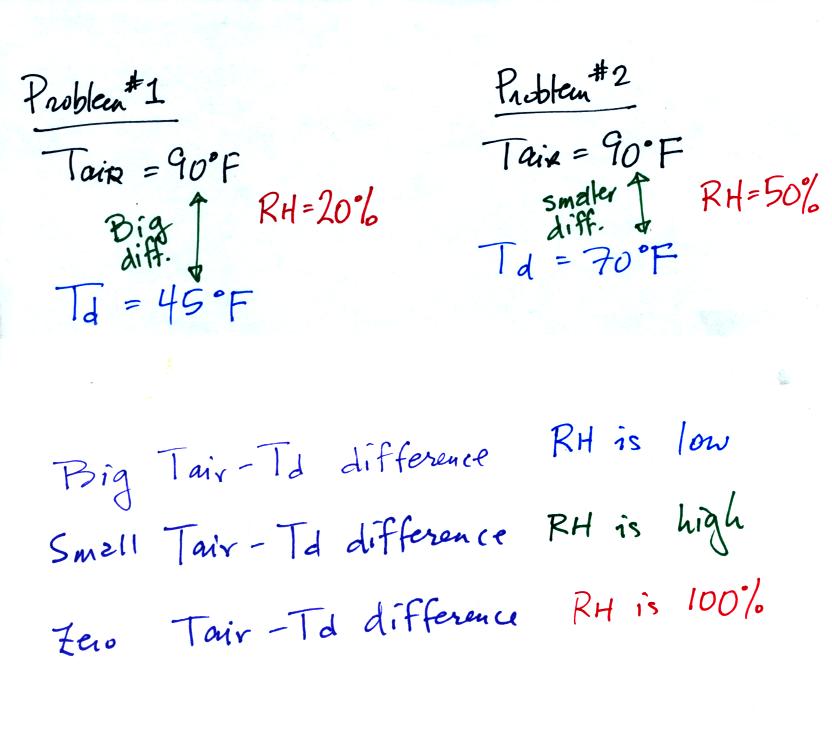
In the first
example the difference between the air and dew point
temperatures was large (45 F) and the RH was low.
In the difference between the air and dew point temperatures was
smaller and the RH was high. The easiest way to remember this
rule is to remember the case where there is no difference between the
air and dew
point temperatures. The RH would be 100%.
Now on to
Problem #3

This figure was redrawn after class. You are given a
mixing ratio
of 10.5 g/kg and a relative humidity of 50%. You need to figure
out the air temperature and the dew point temperature.
(1) The air contains 10.5 g/kg of water vapor, this is 50%,
half, of what the air
could potentially hold. So the air's capacity, the saturation
mixing ratio must be 21 g/kg (you can either do this in your head or
use the RH equation following the steps shown).
(2) Once you know the saturation mixing
ratio you can look up the air temperature in a table.
(3) Then you
imagine cooling the air until the RH becomes 100%. This occurs at
60 F. The dew point is 60 F.
Problem #4 is probably the most difficult of the bunch.
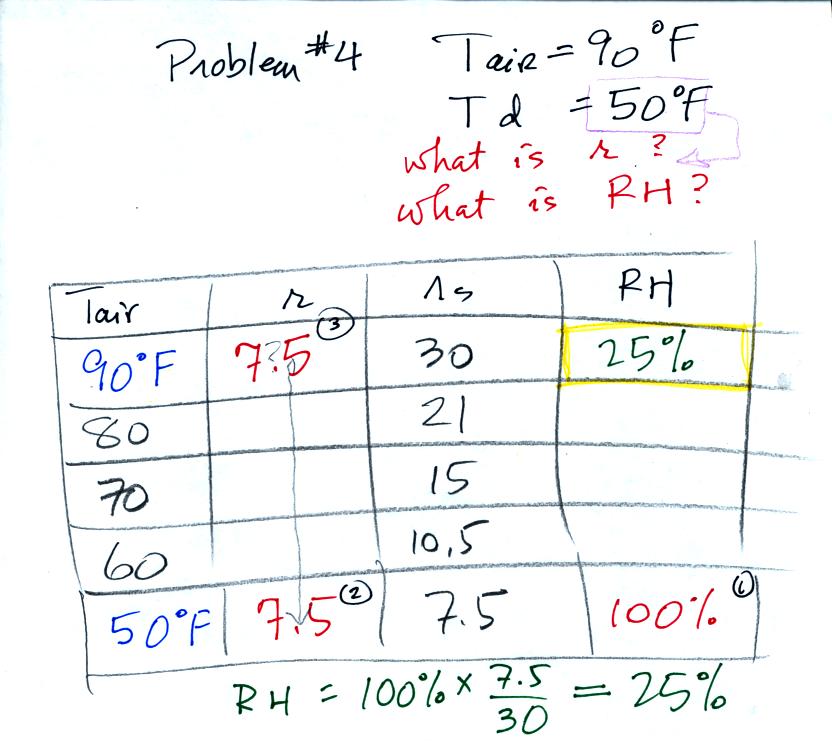
Here's what we did in class. We were given the air
temperature
and the dew point temperature.
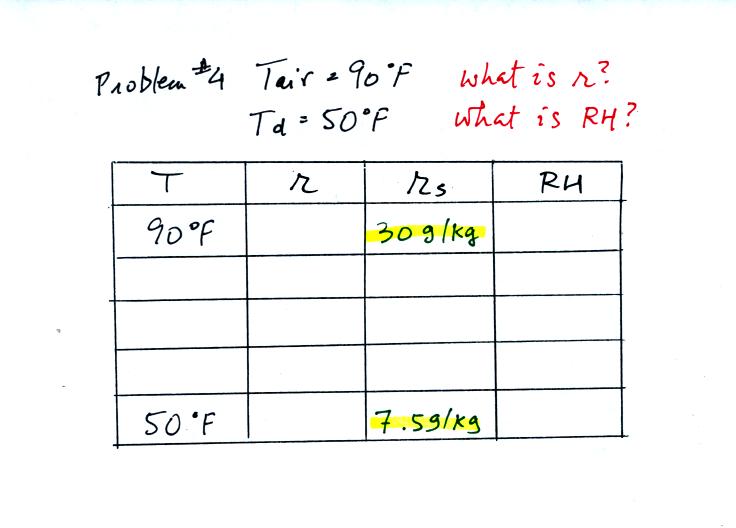
We enter the two temperatures onto a chart and look up the
saturation
mixing ratio for each.
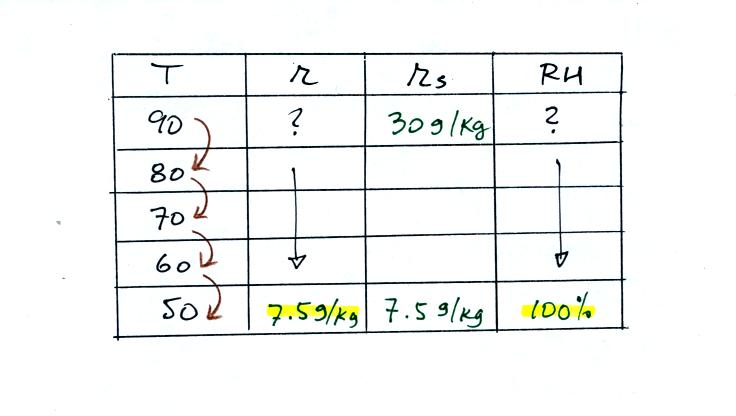
Then we know that if we cool the 90 F air to 50 F the RH
will
become
100%. We don't know the mixing ratio or the relative
humidity. But we do know that when we arrive at 50 F the RH will
be 100%. The mixing ratio must be equal to the saturation mixing
ratio value for 50 F air, 7.5 g/kg.

Remember back to the three earlier examples. When we
cooled air
to the the dew point, the mixing ratio didn't change. So the
mixing ratio must have been 7.5 all along. Once we know the
mixing ratio in the 90 F air it is a simple matter to calculate the
relative humidity, 25%.
Next we
will use what we have learned about humidity
variables (what they tell you about the air and what causes them to
change value) to learn something new.

At Point 1 we start with some 90 F air with a relative
humidity of 25%, fairly dry air (these data are the same as in Problem
#4). Point 2 shows the air being cooled to the dew point, that is
where the relative humidity would reach 100% and a cloud would form.
Then the air is cooled below the dew
point, to
30 F. Point 3 shows the 30 F air can't hold the 7.5 g/kg of water
vapor that
was originally found in the air. The excess moisture must
condense (we will assume it falls out of the air as rain or
snow). When air reaches 30 F it contains less than half the
moisture (3 g/kg) that it originally did (7.5 g/kg). Next, Point
4, the 30
F air is warmed back to 90 F, the starting temperature. The air
now
has a RH of only 10%.
Drying moist air is like wringing moisture from a wet sponge.
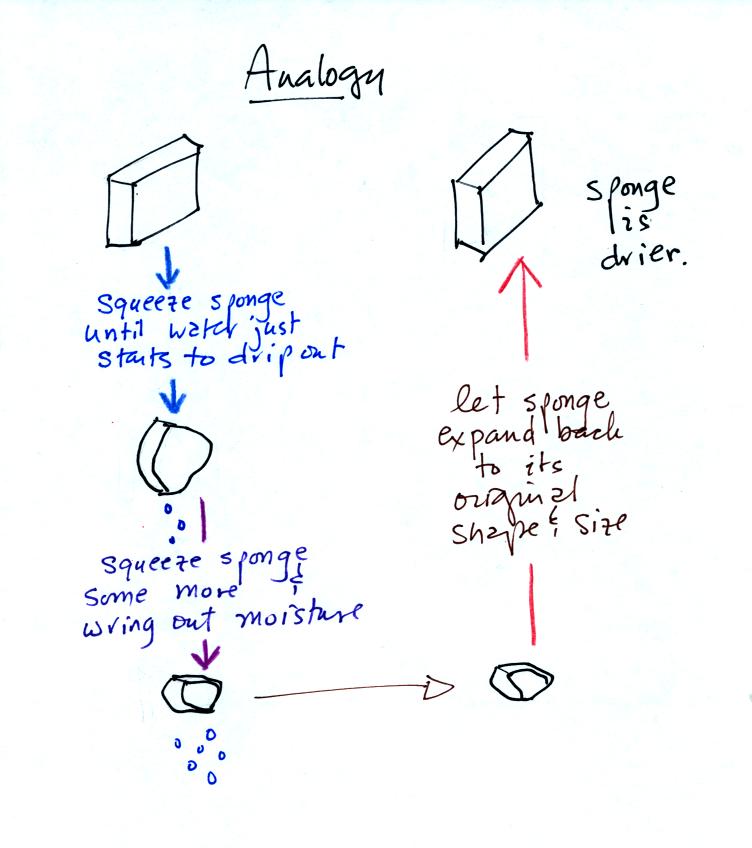
You start to
squeeze the sponge and nothing happens at first (that's like cooling
the air, the mixing ratio stays constant as long as the air doesn't
lose any water vapor). Eventually water will start to drop from
the sponge (with air this is what happens when you reach the dew point
and continue to cool the air below the dew point). Then you let
go of the sponge and let it expand back
to its orignal shape and size (the air warms back to its original
temperature). The sponge (and the air) will be drier than when
you started.
This sort of process ("squeezing" water vapor out of moist air by
cooling the air below its dew point) happens all the time. Here
are a couple of examples.

In the
winter cold air is brought inside your house or apartment and
warmed. Imagine 30 F air with a RH of 100% (this is a best case
scenario, the cold winter air usually has a lower dew point and is
drier). Bringing the air inside and warming it will cause the RH to
drop from 100% to 20%.. Air indoors during the winter is often
very dry.
The air in an
airplane comes from outside the plane. The air outside the plane
can be very cold (-60 F perhaps) and contains very little water
vapor (even if the -60 F air is saturated it would contain essentially
no water vapor). When brought inside and warmed to a
comfortable
temperature, the RH of the air in the plane will be very close
0%.
Passengers often complain of becoming dehydrated on long airplane
flights. The plane's ventilation system probably adds moisture to
the
air so that it doesn't get that dry.
Here's a very important example, the rain shadow effect (the figure in
class was redrawn for clarity).
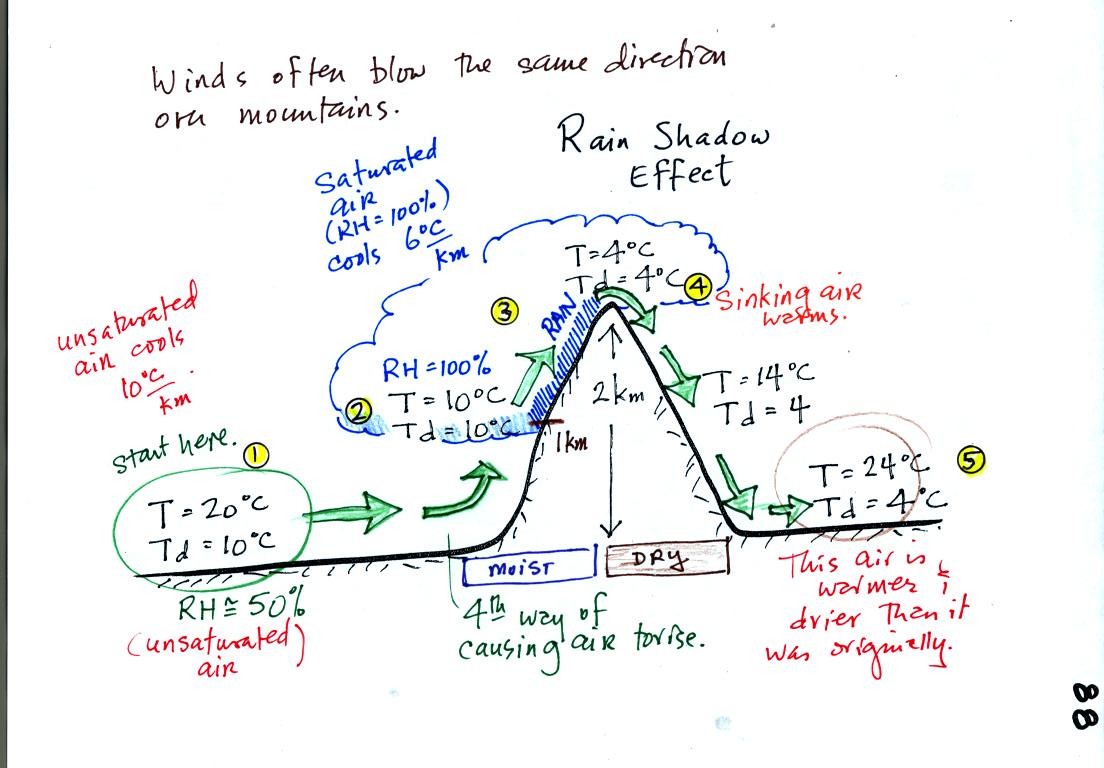
We start with some moist but unsaturated air (RH is about
50%) at Point
1.
As it is moving toward the right the air runs into a mountain and
starts to rise (see the note below). Unsaturated air
cools 10 C for every kilometer of altitude gain.
This is known as the dry adiabatic lapse rate. So in rising 1 km
the air will cool to 10 C which is the dew point.
The air becomes saturated at Point 2, you would see a cloud
appear. Rising saturated air cools at a slower rate than
unsaturated air. We'll use a value of 6 C/km (an average
value). The air cools from 10 C to 4
C in next kilometer up to the top of the mountain. Because the
air is being cooled below its dew point at Point 3, some of the water
vapor will condense and fall to the ground as rain.
At Point 4 the air starts back down the right side of the
mountain. Sinking air is compressed and warms. As soon as
the air starts to
sink and warm, the relative humidity drops below 100% and the cloud
evaporates. The sinking air will warm at the 10 C/km rate.
At Point 5 the air ends up warmer (24 C vs 20 C) and drier (Td =
4 C vs Td = 10 C) than when it started out. The downwind side of
the mountain is referred to as a "rain shadow" because rain is less
likely there than on the upwind side of the mountain. The rain is
less likely because the air is sinking and because the air on the
downwind side is drier than it was on the upslope side.
Most of the year the air that arrives in Arizona comes from the Pacific
Ocean. It
usually isn't very moist by the time it reaches Arizona because it has
travelled up and over the
Sierra Nevada mountains in
California and the Sierra Madre mountains further south in
Mexico. The air loses much of its moisture on the western slopes
of those mountains.
NOTE: The figure
above illustrates orographic or topographic lifting.
It is one of 4
ways of causing air to rise. We have already run into the other
three in class this semester. They were: convergence
(surface winds spiral into centers of low pressure), convection (warm
air rises), and fronts. Rising air is important because rising
air expands and cools. Cooling moist air raises the relative
humidity and a cloud might form.
A sling
psychrometer is a simple instrument that can be used to measure
relative humidity and dew point temperature.
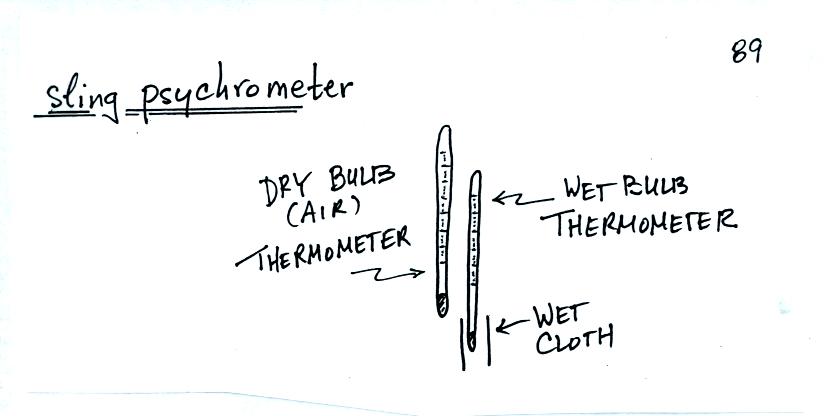
A sling
psychrometer consists of two thermometers mounted
side by side. One is an ordinary thermometer, the other is
covered with a wet piece of cloth. To
make a humidity measurement you swing the psychrometer around for a
minute or two and then read the temperatures from the two
thermometers. The dry - wet buld temperature difference can be
used to determine relative humidity and dew point (see Appendix D at
the back of the textbook).
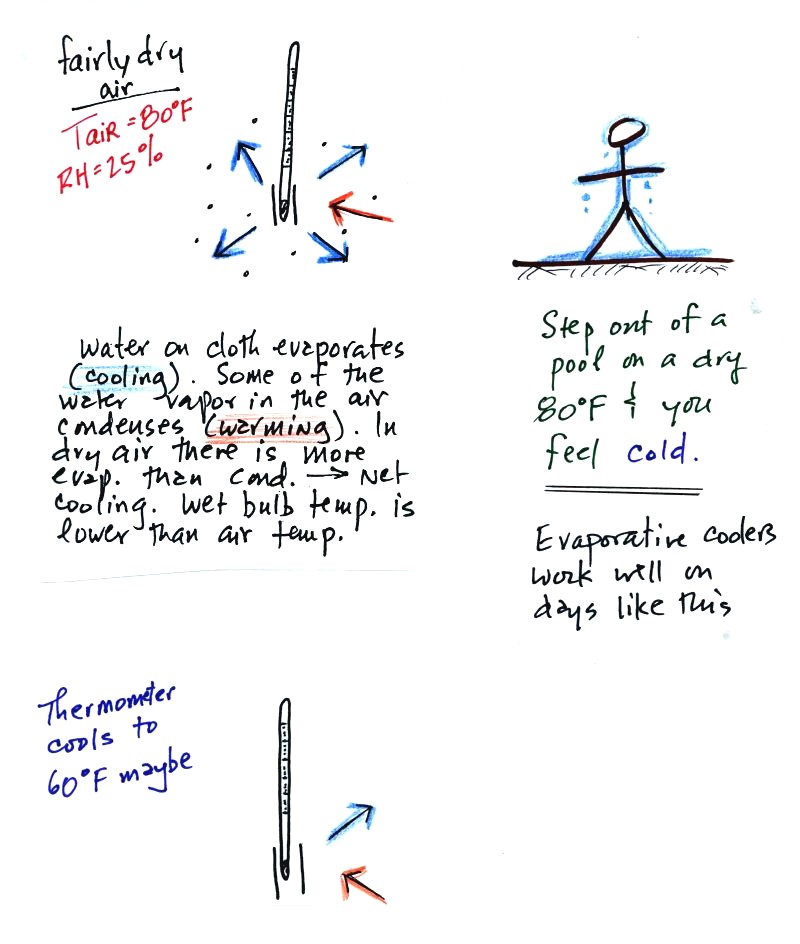
The figure at upper left shows what
will happen as you start to swing the wet bulb thermometer. Water
will begin to evaporate from the wet piece of cloth. The amount
or rate of evaporation will depend on the air temperature (the 80 F
value was just made up in this example).
The evaporation is shown as blue arrows because this will cool the
thermometer. The same thing would happen if you were to step out
of a swimming pool on a warm dry day, you would feel cold. Swamp
coolers would work well on a day like this.
The figure at upper left also shows one arrow of condensation.
The amount or rate of condensation depends on how much water vapor is
in the air surrounding the thermometer. In this case (low
relative humidity) there isn't much water vapor. The
condensation arrow is orange because the condensation will release
latent heat and warm the thermometer.
Because there is more evaporation (4 arrows) than condensation (1
arrow) the wet bulb thermometer will drop.
Note in the bottom left figure we imagine that the wet bulb thermometer
has cooled to 60 F. Because the wet piece of cloth is cooler,
there is less evaporation. The wet bulb thermometer has cooled to
a temperature where the evaporation and condensation are in
balance. The thermometer won't cool any further.
You
would measure a large difference between the dry and wet bulb
thermometers (20 F) on a day like this when the air is relatively dry.
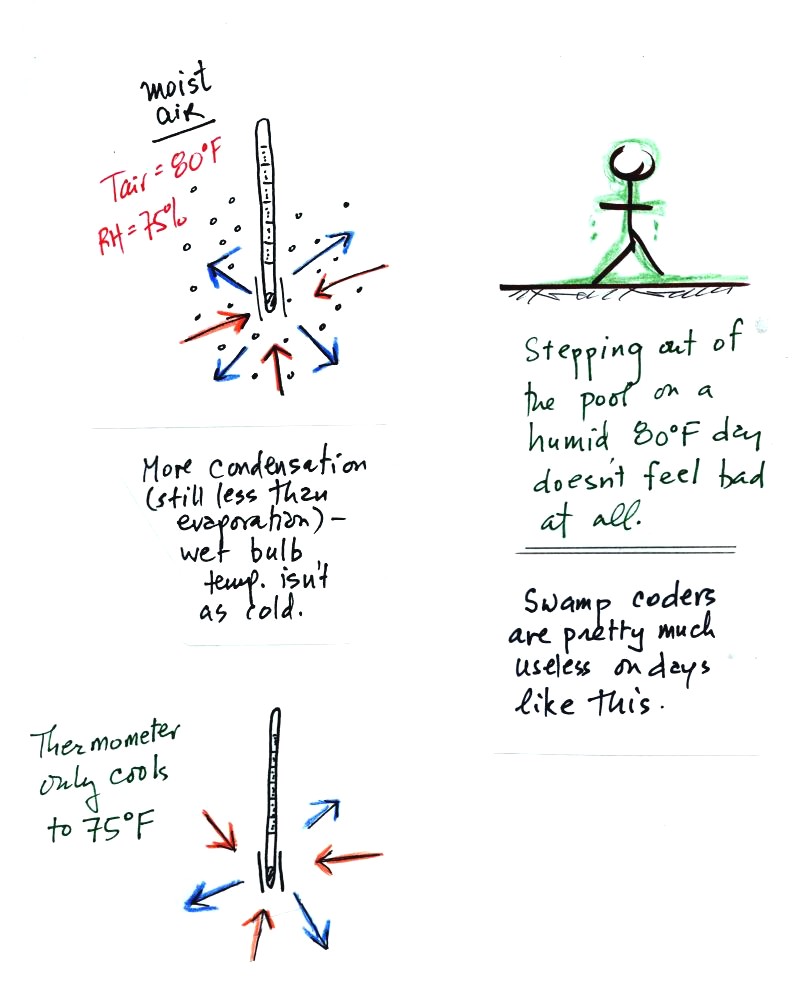
The air temperature is the same in this
example, but there is more
water vapor in the air.
You wouldn't feel as cold if you stepped out of a pool on a warm humid
day like this. Swamp coolers wouldn't provide much cooling on a
day like this.
There are four arrows of evaporation (because the air temperature is
the same as in the previous example) and three arrows now of
condensation (due to the increased amount of water vapor in the air
surrounding the thermometer). The wet bulb thermometer will cool
but won't get as
cold as in the previous example.
The wet bulb thermometer might well only cool to 75 F. This might
be enough to lower the rate of evaporation enough to bring it into
balance with the rate of condensation.
You would measure a small difference (5 F) between the dry and wet bulb
thermometers on a humid day like this one.
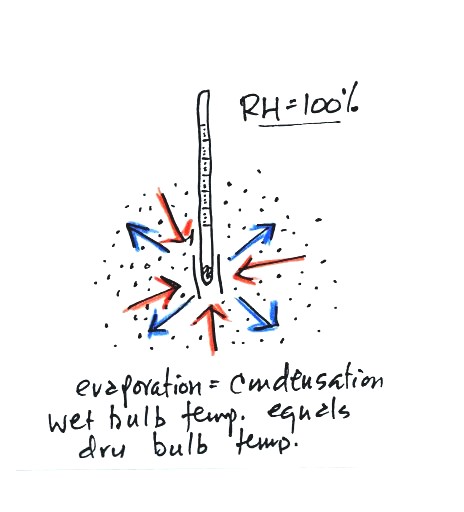
There won't be any difference in
the dry and wet bulb temperatures when
the
RH=100%. The dry and wet bulb thermometers would both read 80 F.

We will be discussing many of the phenomena above. They all
involve cooling air to (and/or below) the dew point temperature.
The air becomes saturated (RH=100%) and water vapor begins to condense.
It turns out that it is much easier for water vapor to condense onto
something rather than just forming a small droplet of pure
water Near the ground water vapor will condense onto cold
objects on the ground (the grass, automobile, and newspaper
above). In air above the ground water vapor condenses onto small
particles in the air called condensation nuclei. We'll learn a
little bit about these today.
The formation of fog and haze, scattering of light, and clouds will all
be covered next Tuesday.
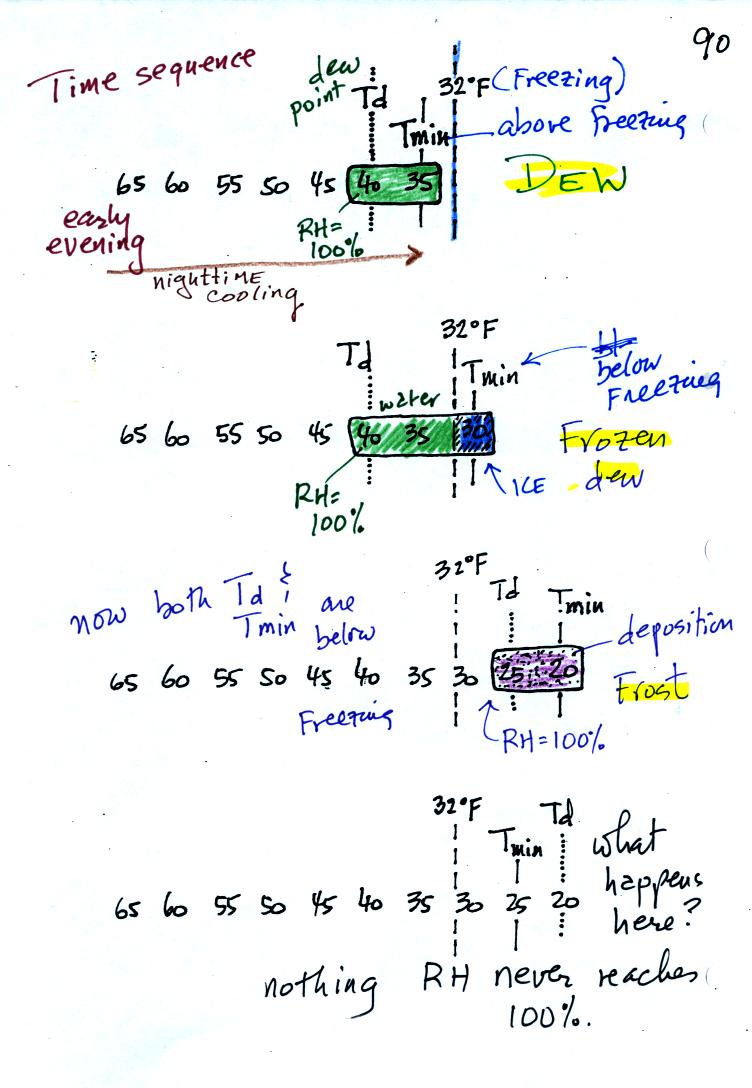
In the first example air starts out with a
temperature
of 65 F early in the evening. It cools to 35 F during the
night. When the air reaches 40 F, the
dew point, the RH reaches 100%. As the air temperature drops
below the dew point and cools to 35 F water vapor will condense onto
the ground or objects on the ground (such as an automobile). This
is dew.
The dew point is the same but the nighttime minimum temperature
is below freezing in the second example. Dew will form again on
this night when the
air temperature reaches 40 F. Once the air temperature drops
below 32 F though the dew will freeze and form frozen dew.
In the third example both the dew point and nighttime minimum
temperatures are below freezing. When the air temperature drops
below the dew point, water vapor turns directly to ice (deposition) and
forms frost.
The dew point in this case is sometimes called the frost point.
The air never becomes saturated in the fourth example because the
nighttime minimum temperature never cools to the dew point. You
wouldn't see anything on this night.
When air
above the ground reaches 100% relative humidity it is much easier for
water vapor to condense onto small particles in the air called
condensation nuclei than to just form a small droplet of water.
There are hundreds even thousands of these small particles in every
cubic centimeter of air. We can't see them because they are so
small.
You can learn why it is so hard to form small droplets of pure water by
reading the top of p. 92 in the
photocopied class notes.

Water vapor will condense onto certain kinds of condensation
nuclei
even when the relative humidity is below 100% (again you will find some
explanation of this on the bottom of p.
92). These are called hygroscopic
nuclei.
A short video showed how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of glass.

The start of the video at left showed the small grains of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right). The figure above wasn't shown in class.
In humid parts of the US, water will condense onto the grains of salt
in a salt shaker causing them to stick together. Grains of rice
apparently will keep this from happening and allow the salt to flow
freely out of the shaker when needed.



























