Friday Oct. 31, 2008
click here to download today's notes in
more printer friendly Microsoft WORD format
Sorry I couldn't really find any Halloween music that I liked and had
to fall back on an old standby, Rodrigo y Gabriela.
The song was called Vikingman.
All three parts of the Quiz #3 Study Guide are now online (here, here,
and here). Times and locations
of the reviews are on Pt. 3 of the study guide.
The Experiment #3 reports are due next
Monday together with the 1S1P Assignment #2
reports. If you haven't brought back your experment materials and
picked up the supplementary information sheet it is too late now.
Class began with a quick review of the two processes that are able
to quickly turn small cloud particles into much larger precipitation
particles, the Collision Coalescence process and the Ice Crystal
Process. This information was stuck onto the end of the Wed., Oct. 29 notes. With that and an
appeal (and a threat) for quiet and full attention to the new material
that needed to be covered in class, we began with the ice crystal
process. This is somewhat more difficult to understand than the
collision coalescence process.
Before
learning the ice
crystal process, we need to first look at the structure of cold
clouds.
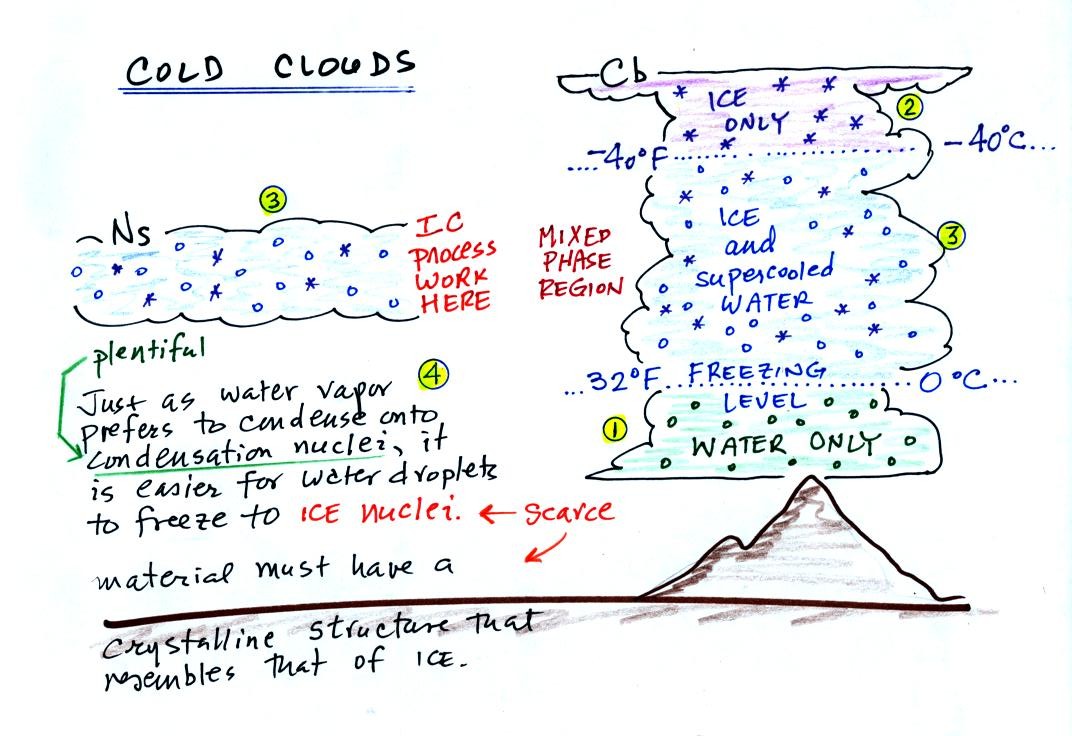
The figure below is a redrawn version of what was drawn in class.
The bottom of the thunderstorm, Point 1, is warm
enough
(warmer than freezing) to just
contain water
droplets. The top of the thunderstorm, Point 2, is colder than
-40 C and just contains ice crystals. The interesting part of the
thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains both
supercooled water
droplets (water that has
been cooled to below freezing but hasn't frozen) and ice
crystals.
This is called the mixed phase
region. This is where the ice crystal process will be able
to produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
The supercooled water droplets aren't able to freeze even though
they
have been cooled below freezing. At Point 4 we see this is
because it is much
easier for small droplets of water to freeze onto an ice crystal
nucleus or for water vapor to be deposited onto an ice crystal nucleus
(just like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small droplet
of pure water). Not just any material will work as an ice nucleus
however. The material must have
a crystalline structure that is like that of ice.
We'll see
next how the ice crystal process works. There are a couple of
"tricky" parts.
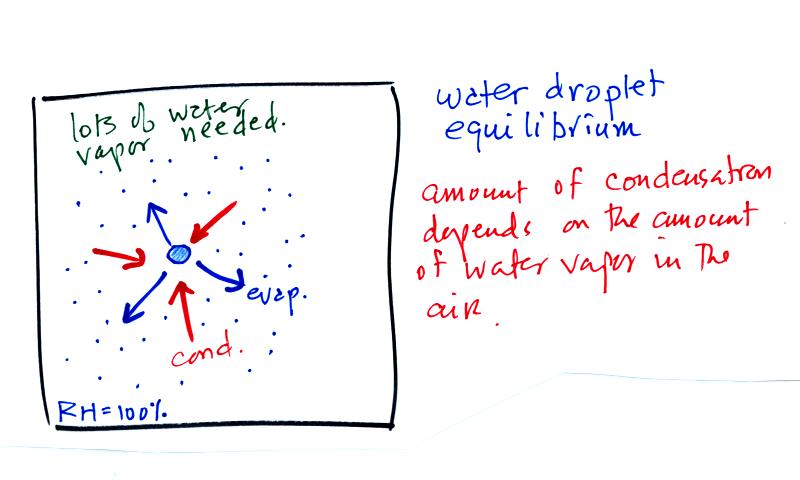
The first figure above (see p.101 in the photocopied
Class
Notes)
shows a water droplet in equilibrium with its surroundings..The droplet
is evaporating (the 3 blue arrows in the figure). The rate of
evaporation will depend on the temperature of the water droplet.
The droplet is surrounded by air that is saturated with water vapor
(the droplet is inside a cloud where the relative humidity is
100%). This means there is enough water vapor to be able to
supply 3 arrows of condensation. Because the droplet loses and
gains water vapor at equal rates it doesn't grow or shrink.
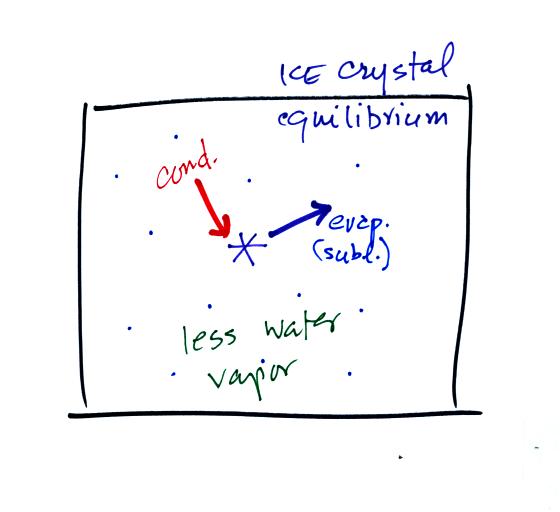
This figure shows what is required
for an ice crystal (at
the same
temperature) to be in equilibrium with its surroundings. First
the ice crystal won't evaporate as rapidly as the water droplet (only
one arrow is shown). Going from ice to water vapor is a bigger
jump than going from water to water vapor. There won't be as many
ice molecules with enough energy to make that jump. A sort of
analogous situation is shown in the figure below. The class
instructor could with a little warmup and practice jump from the floor
up and onto the seat of a chair (maybe 15 inches tall). Trust me,
he could, and so could most of the people in the room. The class
instructor does some stupid things in class, but he wouldn't begin to
consider trying to jump from the floor up to the top of the cabinet (30
inches or more).
To be in equilibrium only one arrow of condensation is
needed.
There doesn't need to be as much water vapor in the air surrounding the
ice crystal to supply this lower rate of condensation.
There are going to be fewer people able to make the
big jump on
the
left just as there are fewer ice molecules able to sublimate.
Going from water to water vapor is a "smaller jump" and more molecules
are able to do just as more people would be able to make the shorter
jump at right in the picture above.
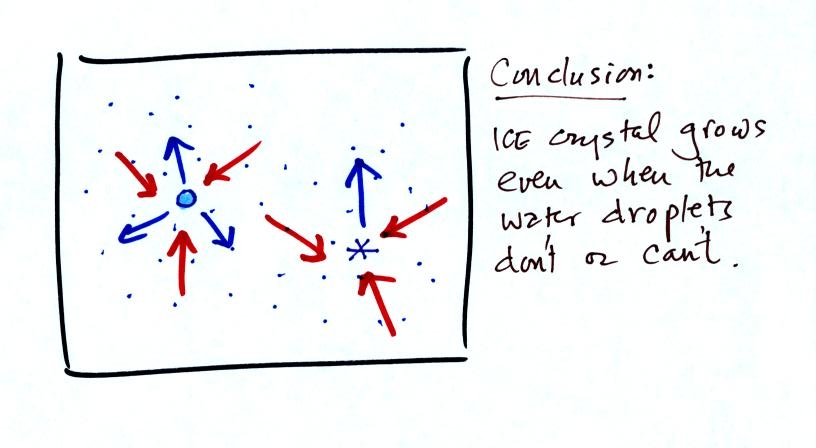
Now what happens in the mixed phase region of a cold cloud
is that
ice crystals find themselves in the very moist surroundings needed for
water droplet equilibrium. This is shown below.
The water droplet is in equilibrium (3 arrows of evaporation
and 3
arrows of condensation) with the surroundings. The ice crystal is
evaporating more slowly than the water droplet. Because the ice
crystal is in the same surroundings as the water droplet water vapor
will be condensing onto the ice crystal at the same rate as onto the
water droplet. The ice
crystal isn't in equilibrium, condensation
exceeds evaporation and the ice crystal will grow. That's
what makes the ice crystal process work.
The equal rates of condensation are shown in the figure
below using the
earlier analogy.
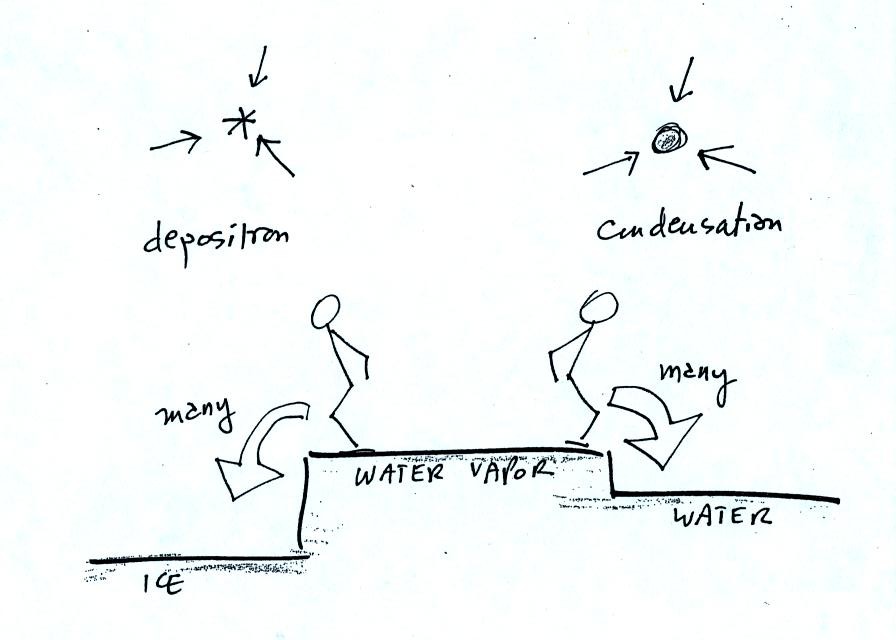
Even though he was afraid to try to jump up to the top of
the counter, the instructor could jump from the counter to the floor.
That's where we left the ice crystal process for today. We'll
come back to it again on Monday.
The computer grade summaries had made their way around the room by
this point. Here's an example of what they look like.

2. The number of extra credit points (from the optional assignments)
that you have earned so far.
3. If you have completed an experiment report, the grade should appear
here. If not, then an average grade was used during the grade
estimate calculations to show the effect of the writing grade on your
overall average. Don't get the idea that you don't have to do any
experiment report, you do. If you don't do a report by the end of
the semester you really will have a zero here.
4. This is the grade you received on your Bonus 1S1P report on Radon.
5. Because some of the reports haven't been graded yet, none of the
1S1P Assignment #1 grades have been used in the grade summary.
Instead the computer "guessed" at how many 1S1P pts you will have at
the end of the semester. If you have turned in two Assignment #1
reports so far, the computer assumed you would continue writing reports
and would earn 45 pts by the end of the semester (45 is the maximum
number of 1S1P pts you can earn during the semester). If you only
turned in 1 report, the computer assumed you would end up with 35
pts. And if you haven't turned in any 1S1P reports, the computer
started to wonder about that and assumed you might have decided not to
write very many 1S1P reports.
It is important to understand that even if you haven't
written any 1S1P reports yet, there is still time to catch up and earn
45 pts (the maximum number of points allowed). But you need to
get started now. The next assignment is due next week and you
should plan on turning in two reports.
It is also important to realize that the number of 1S1P
pts you have been given by the computer for the purposes of this grade
estimate aren't real points. At the end of the semester the
computer will only use points that you have actually earned on reports
that you have turned in.
6. The writing score percentage grade is obtained by adding the
experiment report points (maximum of 40 pts) and the 1S1P pts (maximum
of 45 pts), dividing by 80, and multiplying the result by 100%.
7. This is the computer's guess at what you overall average
grade will be at the end of the semester if you keeping doing as you've
done so far. The average is based on quiz scores and your writing
percentage grade. The extra credit points have been added
in. The first average (no quiz grades dropped) is the one that
has to be 90.0 or above to get out of the final.
Please check your grade report and make sure the grades are
correct. If you have any questions then come and check with me.
Now back to the Collision Coalescence process.
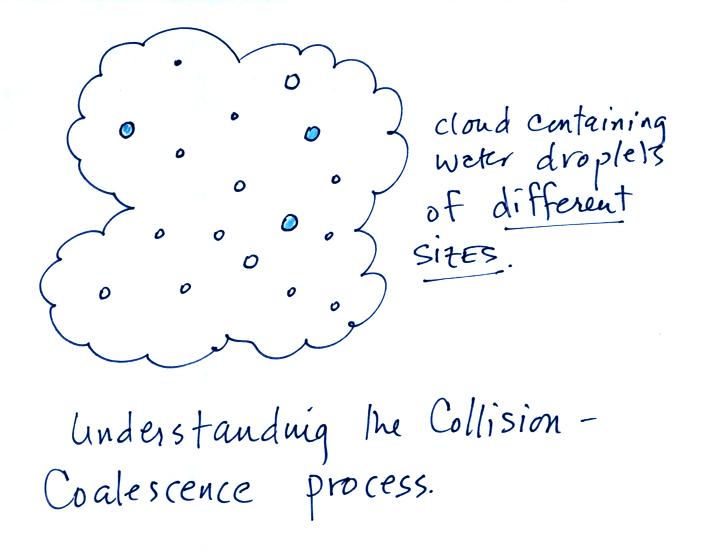
Here's
what you might see if you looked inside a warm cloud with just water
droplets:
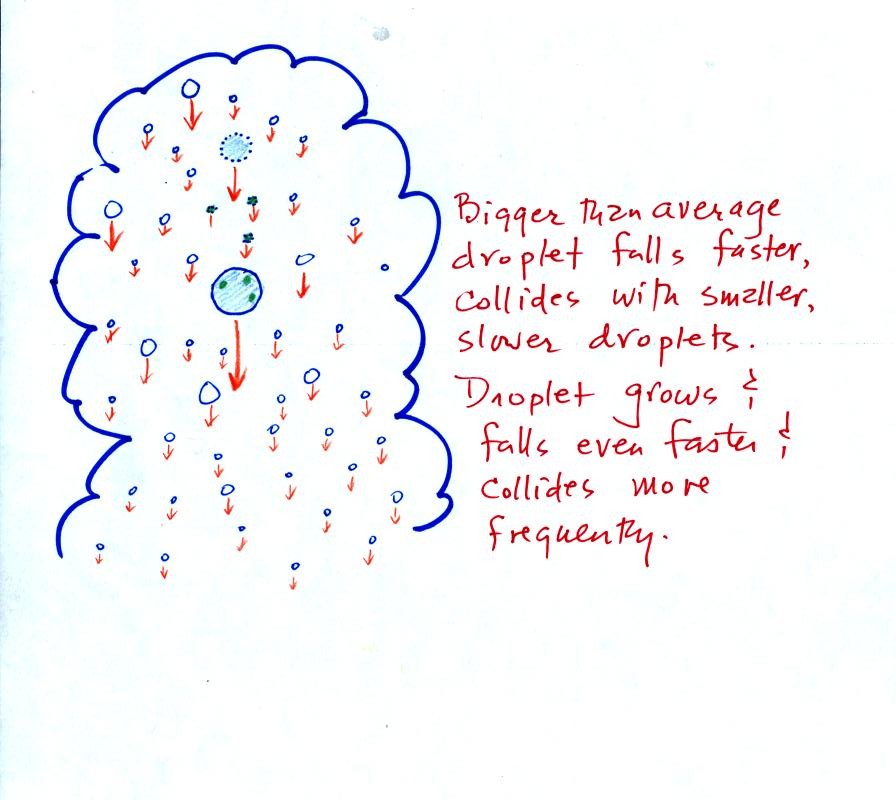
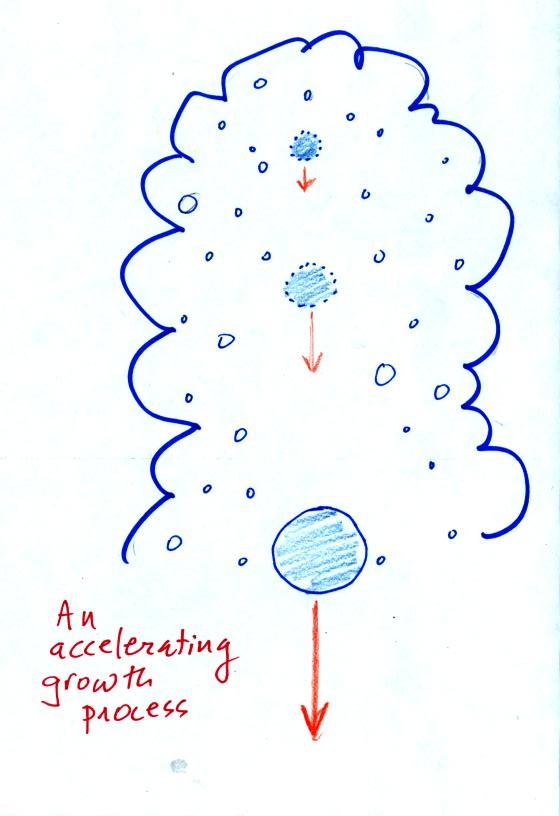
The collision coalescence process works best in a cloud
filled with cloud droplets of different sizes. The larger
droplets overtake and collide with the smaller ones. The droplets
coalesce (stick together), the droplet grows, begins to fall faster,
and collides more frequently with smaller droplets.
This is an acclerating growth process.
The
falling droplet
gets
wider, falls faster, and sweeps out an increasingly larger volume
inside the cloud. The bigger the droplet gets the faster it
starts to grow.
The figure
below shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). A little more carefully drawn
version than was done in class. Ns clouds are thinner
and have weaker updrafts than Cb clouds. The largest raindrops
fall from Cb clouds because the droplets spend more time in the cloud
growing. In a Cb cloud raindrops can grow while being carried upward by
the updraft and also when falling in the downdraft.

Raindrops grow up to about 1/4 inch in diameter.
When
drops get
larger than that, wind resistance flattens out the drop as it falls
toward the ground. The drop begins to "flop" around and breaks
apart
into several smaller droplets. Solid precipitation particles such
as hail can get much larger (an inch or two or three in diameter).
Thanks for cooperating and keeping the chaos don't to a tolerable even
agreable level. I was able to leave class with the feeling that
we had accomplished something.