Wednesday Sept. 10, 2008
Click here for a printer friendly
version of these notes in Microsoft WORD format
We had time for a couple of songs from the Fleet Foxes before class
today.
Here's a new Reading Assignment.
Basically you should finish reading Chapter 1 (you don't need to do it
all at once, we will be covering this material this week and much of
next week). You might also have a look at Appendix C (pps
525-529).
You would
have the whole period if there were a real quiz today. However,
since there is just a practice quiz we spent the first 20 minutes of
class on some new material.
We are going to move
into the middle part of Chapter 1 and start some new, completely
different, material. We will be looking
at how atmospheric characteristics such
as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we first need to understand
what pressure is and what can cause it to change.
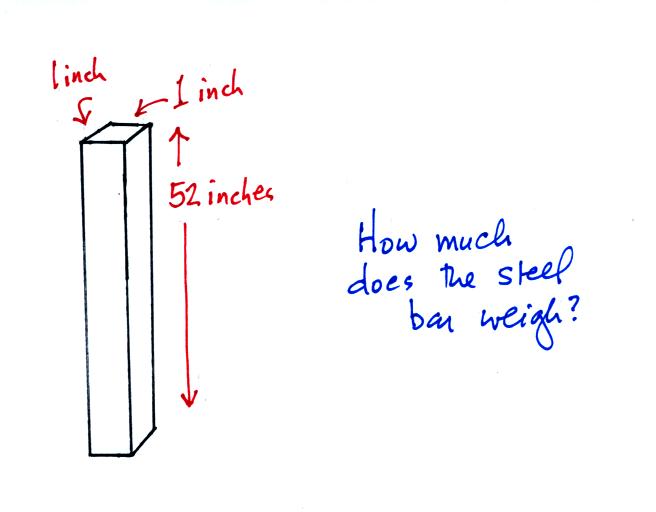
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We will come back to the iron bar in class on Friday.
What
follows is a little more detailed
discussion of the basic concepts of mass, weight, and density
than was covered in class.
Before we can learn about
atmospheric pressure, we
need to review
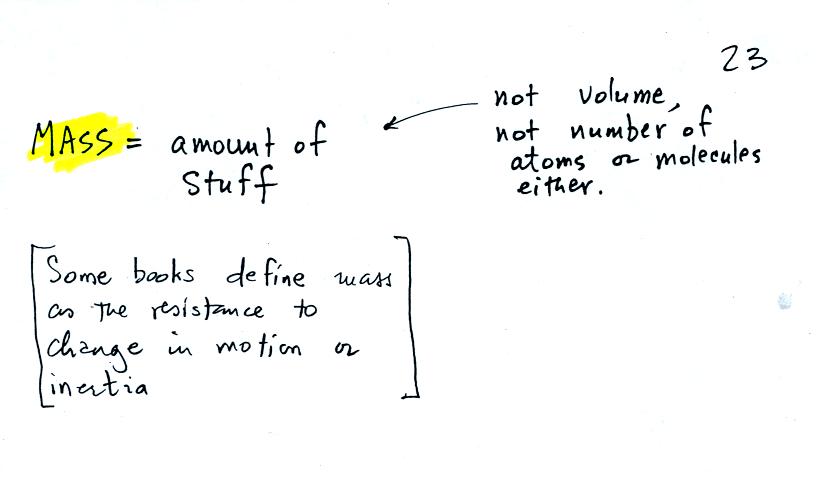
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
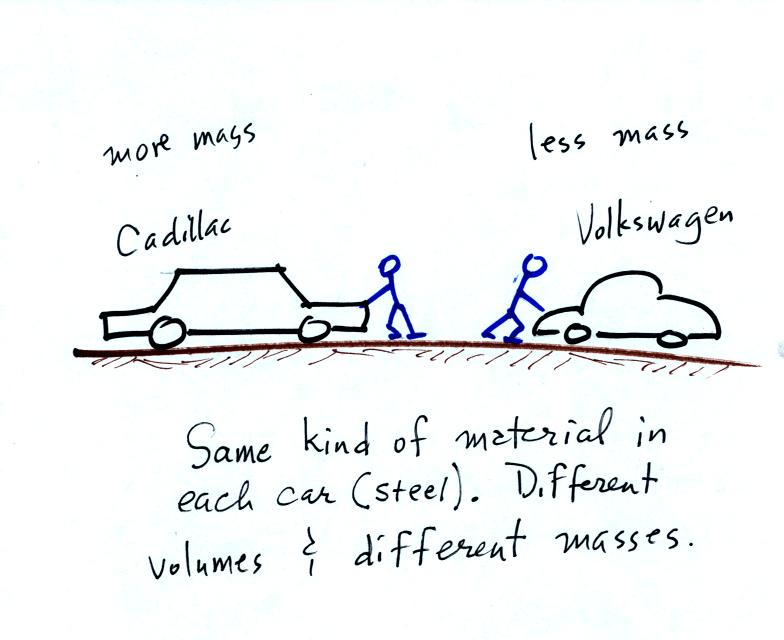
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down if it were
already moving).
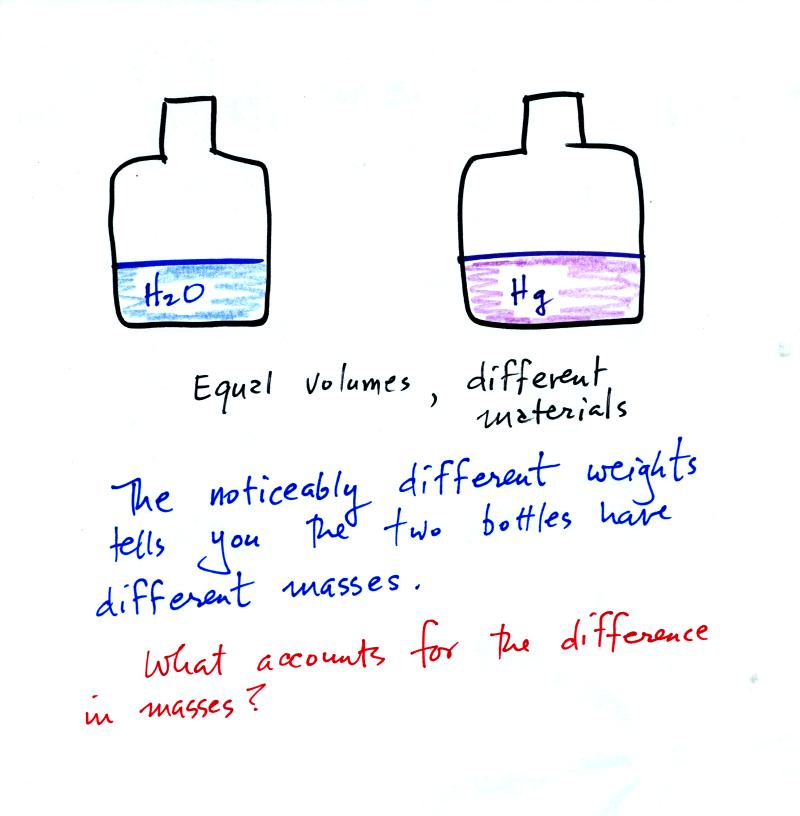
It is possible to have two objects with the
same
volume but very
different masses. The bottles of water and mercury that were
passed around class were an example (thanks for being so
careful
with the mercury).
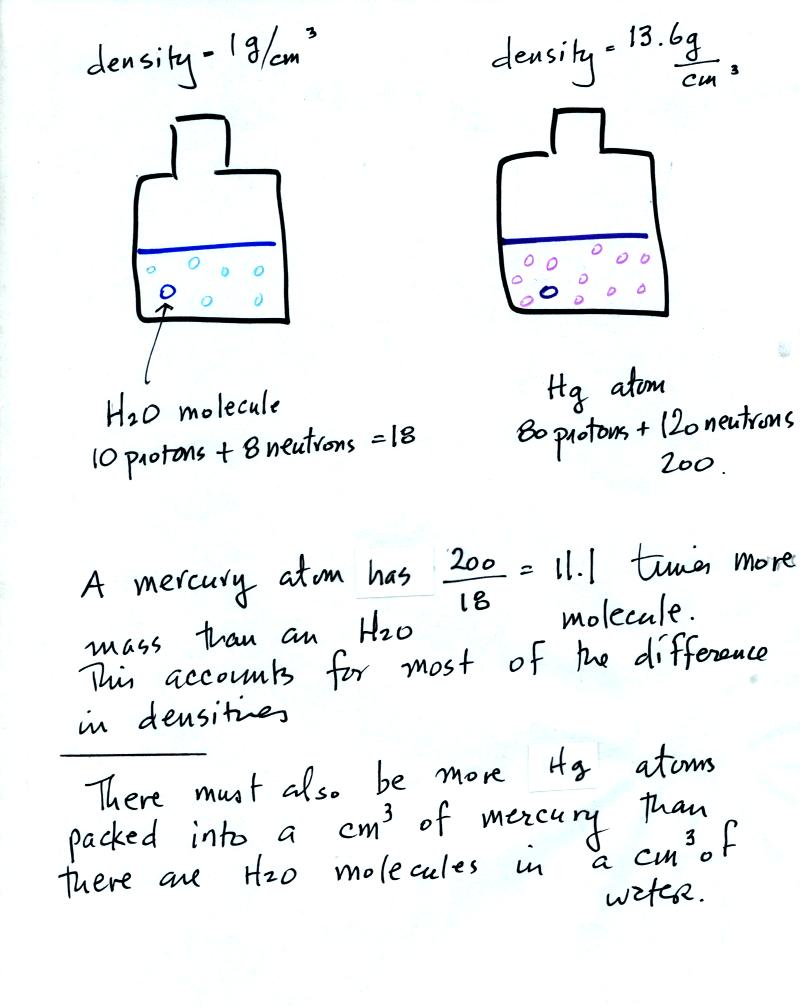
To understand why
there is such a
difference in mass and weight you need to look at the water molecules
and mercury atoms on an atomic scale.
Mercury atoms are built up of many
more protons and neutrons
than a water molecule (also more electrons but they don't have nearly
as much mass as protons and neutrons). The mercury atoms have
11.1 times as much mass as the water molecule. This doesn't quite
account for the 13.6 difference in density. Despite the fact that
they contain more protons and neutrons, the mercury atoms must also be
packed closer together than the molecules in water.
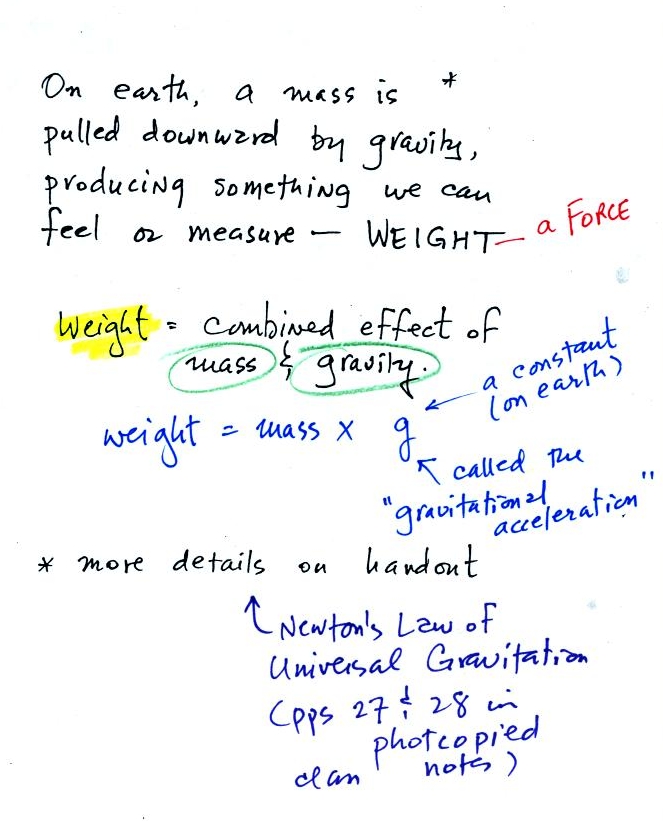
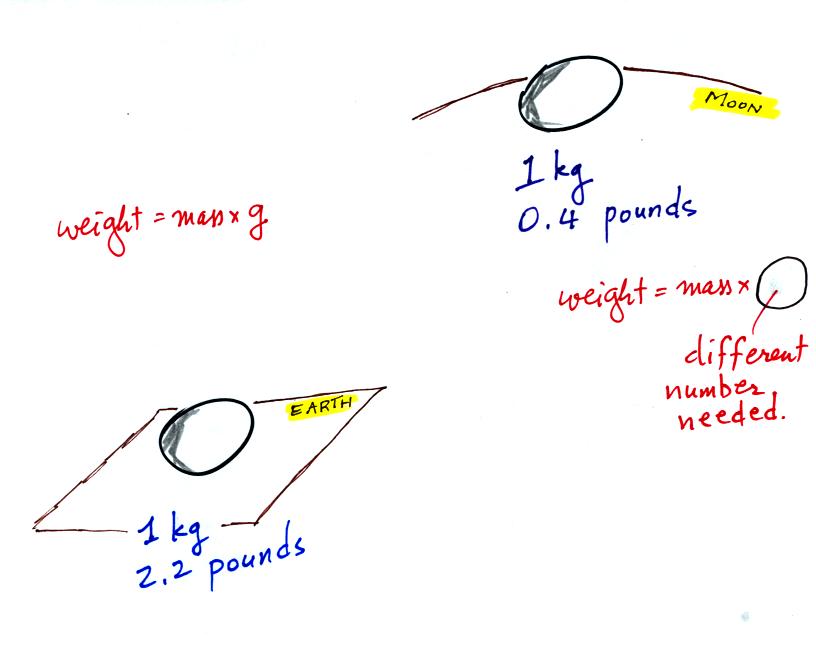
Weight
is a force and depends on
both the mass of an object and the
strength of gravity. We tend to use
weight and mass
interchangeably
because we spend all our
lives on earth where gravity never changes.
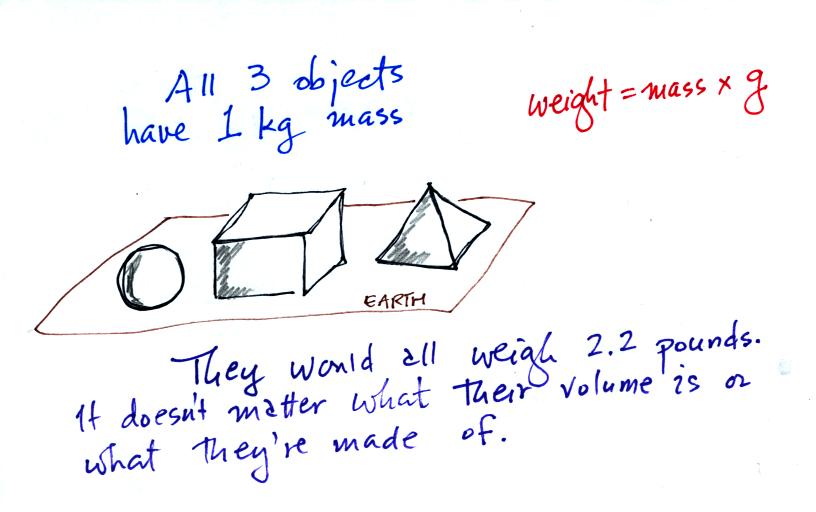
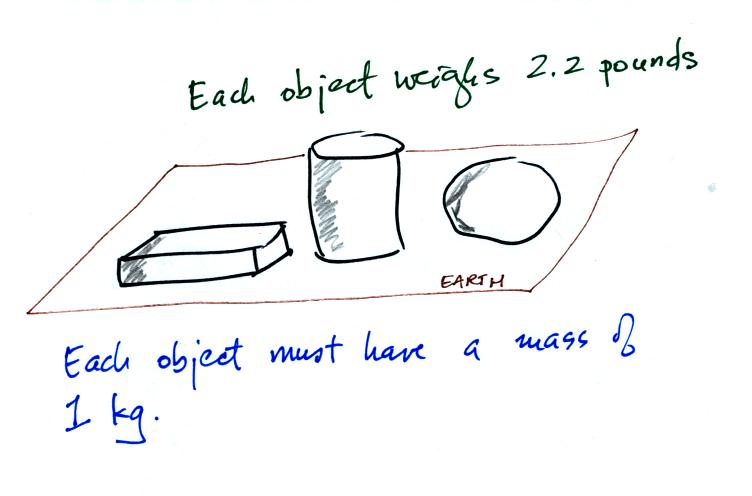
Any three objects that all have the same mass
(even if they had different volumes and were made of different
materials) would
necessarily have the same weight. Conversely
Three
objects with the
same weight
would also have the same mass.
The difference between mass and weight is clearer
(perhaps) if you
compare the situation on the earth and on the moon.
If you
carry an object
from the
earth to the moon, the mass
remains the
same (its the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.
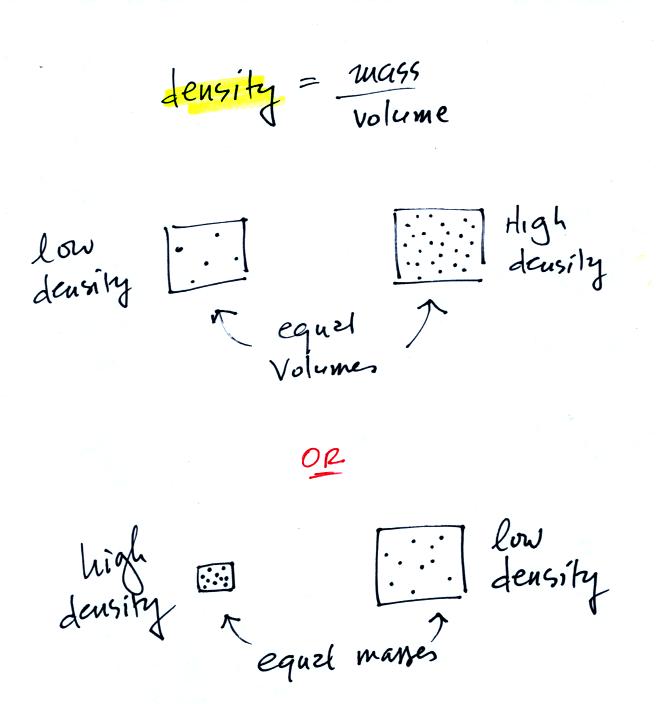
In
the first example there is more mass (more dots) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density.