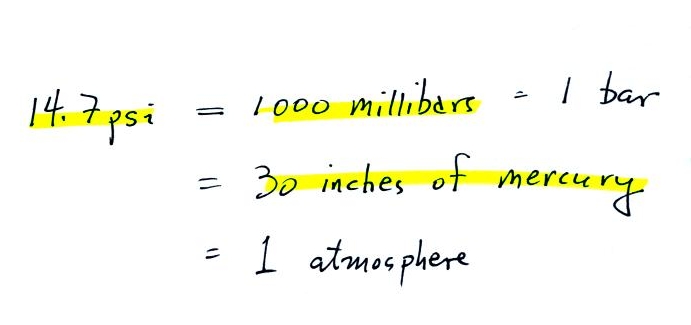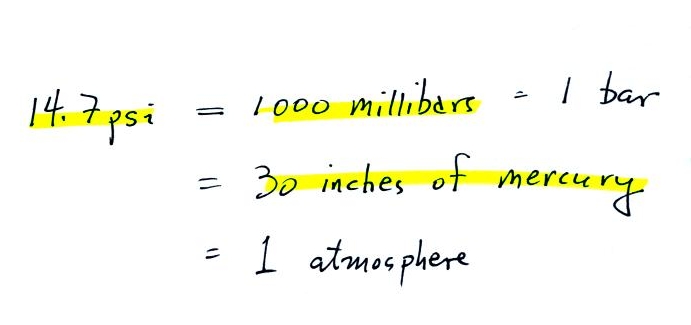
(1) After a long cold winter and
without much bicycling or other exercise, the course instructor
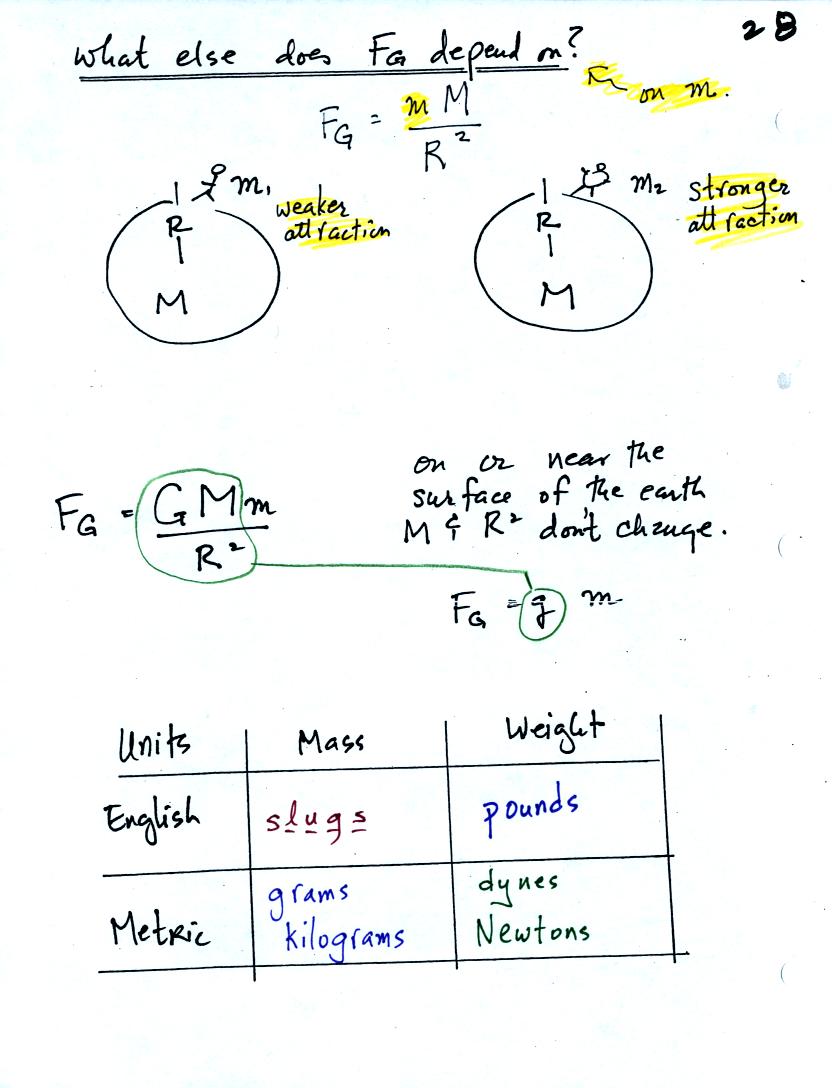
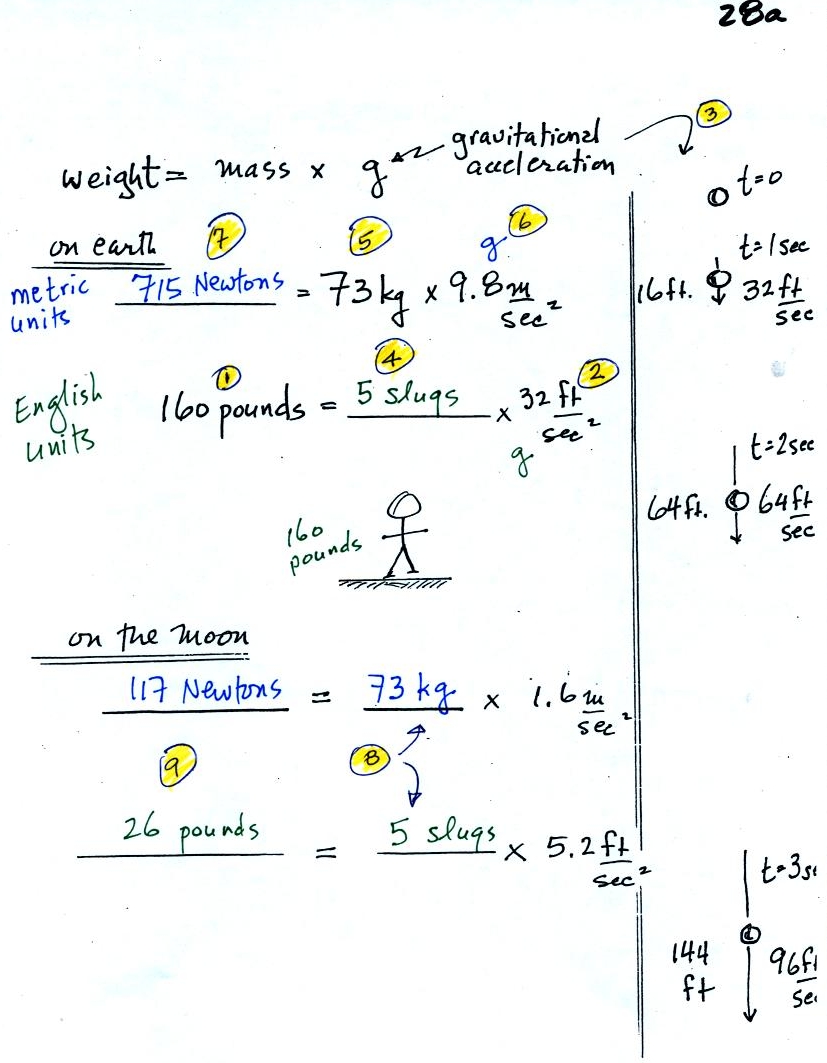
sometimes weighs as much as 160 pounds. In
(2) we see that the
gravitational acceleration (g) is 32 ft/sec2 in English units (on the
earth). The
meaning of this value is shown in (3). If you drop an object it
will start to fall and will speed up as it continues to fall.
Gravity will cause a
falling object to fall 32 ft/sec faster every second. Dividing
the instructor's weight by the gravitation
acceleration in (4) we obtain the instructor's mass, 5 slugs, in
English units.
In metric units, the instructor has a mass of 73 kilograms
(5).
The gravitation acceleration in metric units is 9.8 m/sec (6).
Multiplying these
two values, in (7), we find that the instructor weighs 715 Newtons.
On the moon, the mass stays the same. Gravity is weaker, so
the
value of g is smaller. The instructor would weigh quite a bit
less (117 Newtons or 26 pounds) on the moon compared to the earth.
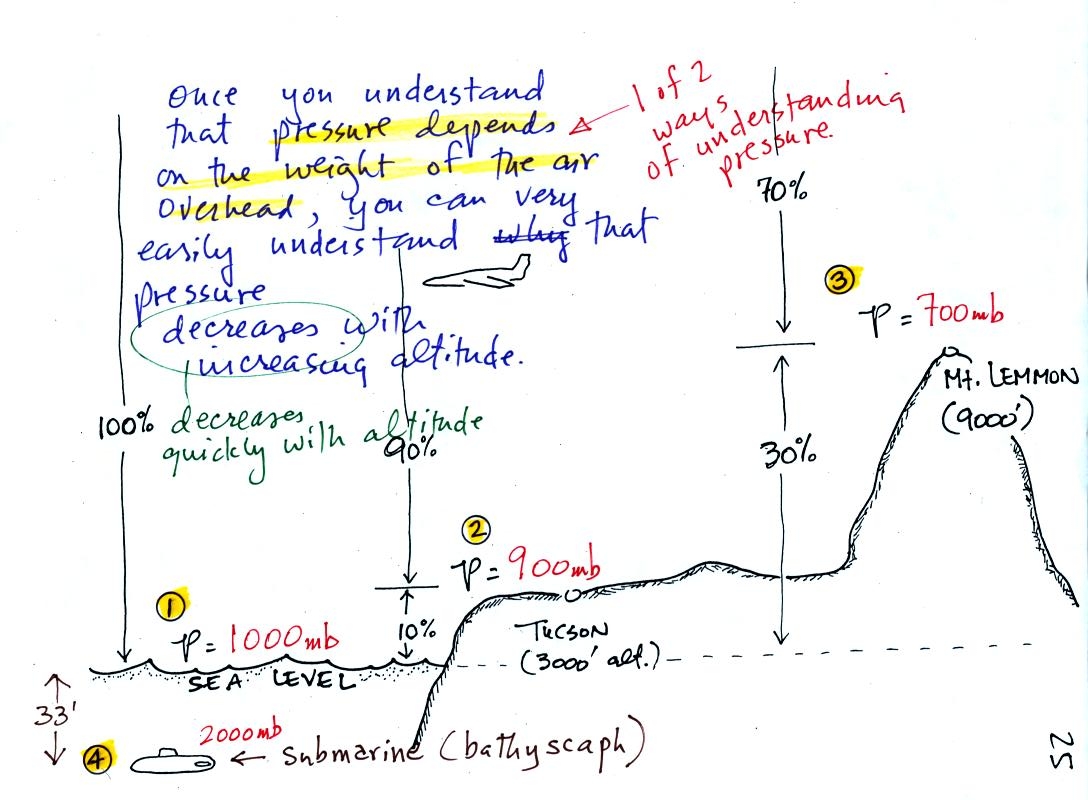
Mercury
barometers are used to measure atmospheric pressure. A mercury
barometer is really just a balance that can be used to weigh the
atmosphere. A basic understanding of how a mercury barometer
works is something that every college graduate should have.
You'll find most of what follows on p. 29 in the
photocopied Class Notes. The figures below are much more
carefully drawn versions of what was shown in class.
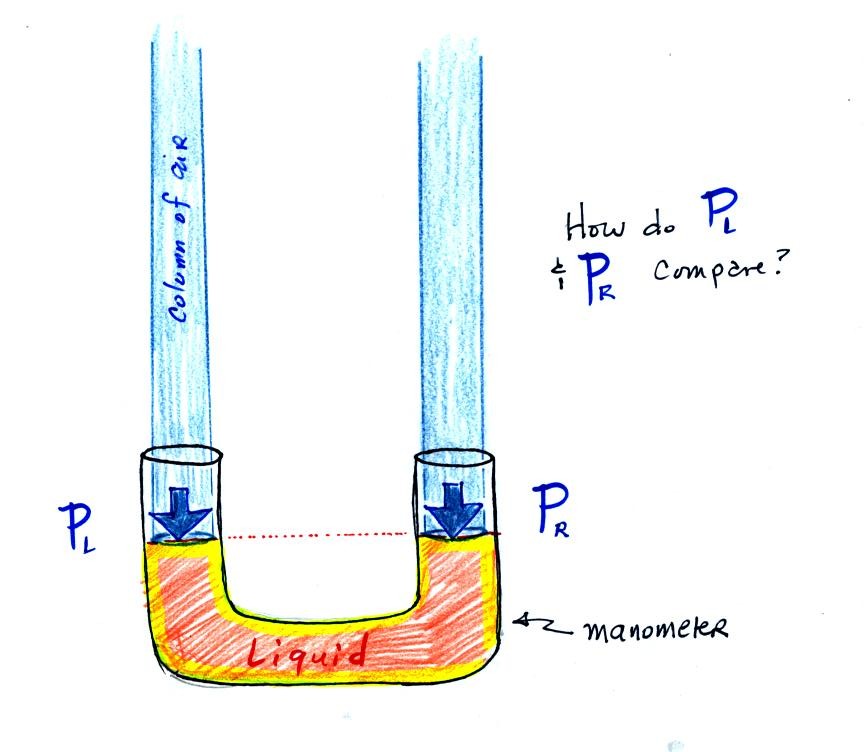
The instrument above ( a u-shaped
glass
tube filled with a
liquid of some kind) is a manometer and can be used to measure pressure
difference. The
two ends of the tube are open so that air can get inside and air
pressure can press on the liquid. Given that the liquid levels on
the two sides of the manometer
are equal, what could you about PL and PR?
The liquid can slosh back and
forth just like the pans on a balance can move up and down. A
manometer really behaves just like a pan balance.
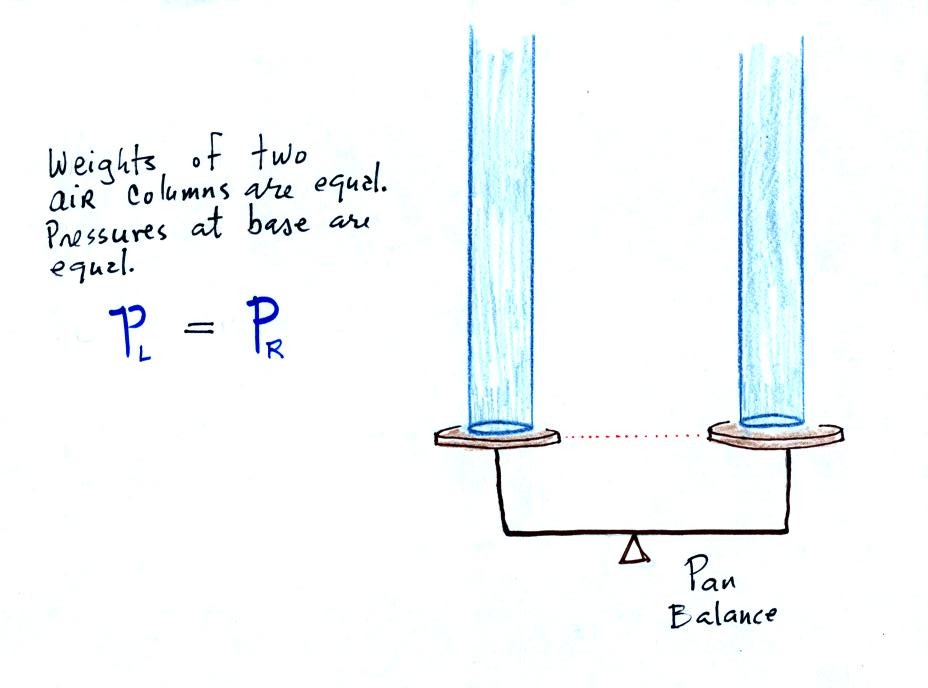
PL and PR
are equal (note
you don't really know what either pressure is just that they are equal).
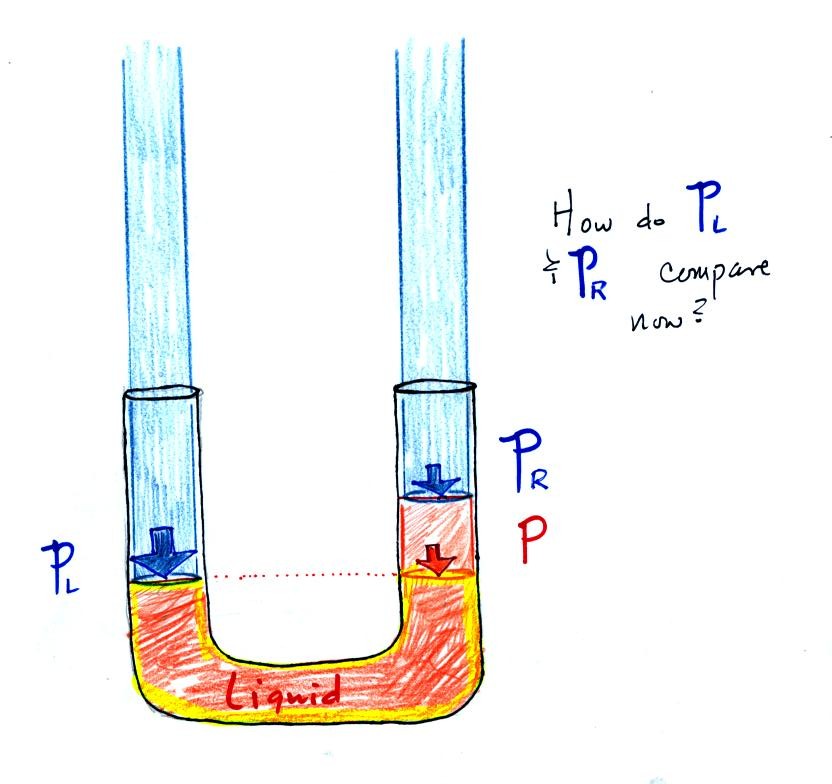
Now the situation is a little
different,
the
liquid levels
are no
longer equal. You probably realize that the air pressure on the
left, PL, is a little higher than the air pressure on the
right,
PR. PL is now being balanced by PR
+ P acting together. P
is the pressure produced by the weight of the extra fluid on the right
hand side of
the manometer (the fluid that lies above the dotted line). The
height of the column of extra
liquid provides a measure of the difference between PL and PR.
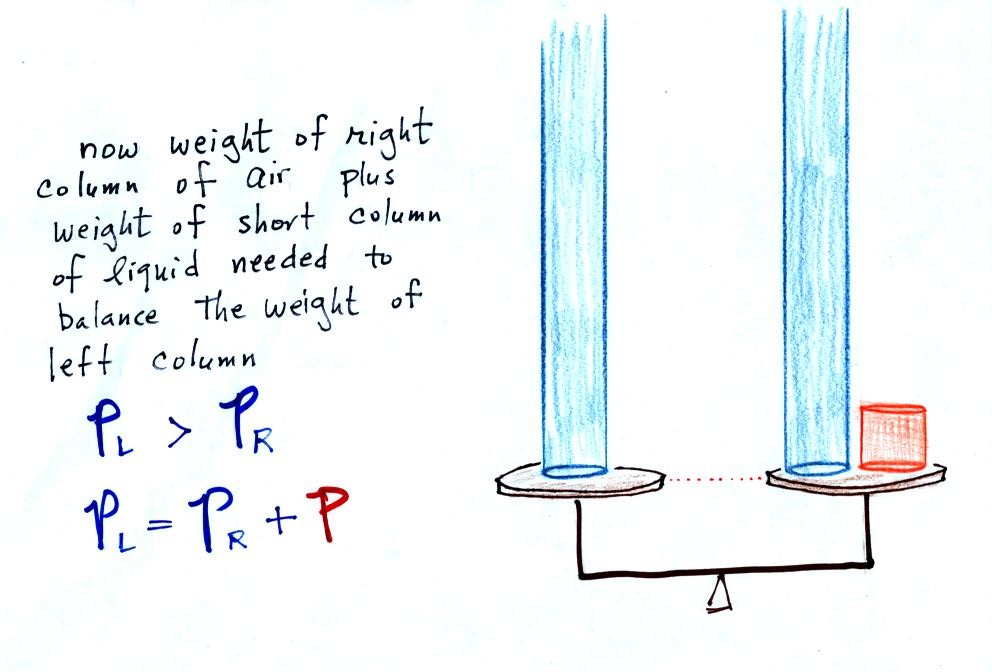
Next we will go an extreme and
close off the right hand side of the
manometer.
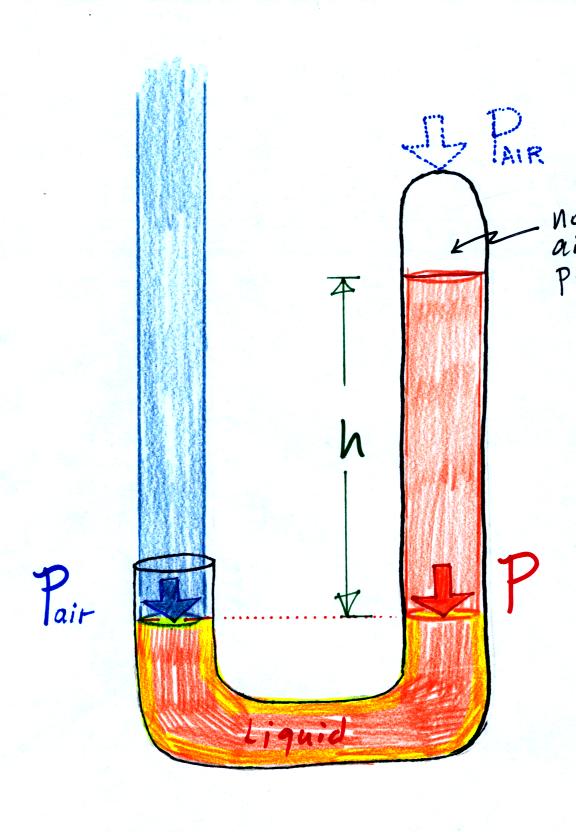
Air pressure can't get into the
right tube any
more. Now at the level of the dotted line the balance is between
Pair and P (pressure by the extra liquid on the
right). If
Pair changes, the height of the right column, h, will
change. You now have a barometer, an instrument that can measure
and monitor the atmospheric pressure. (some of the letters were cut off
in the upper right portion of the figure, they should read "no air
pressure")
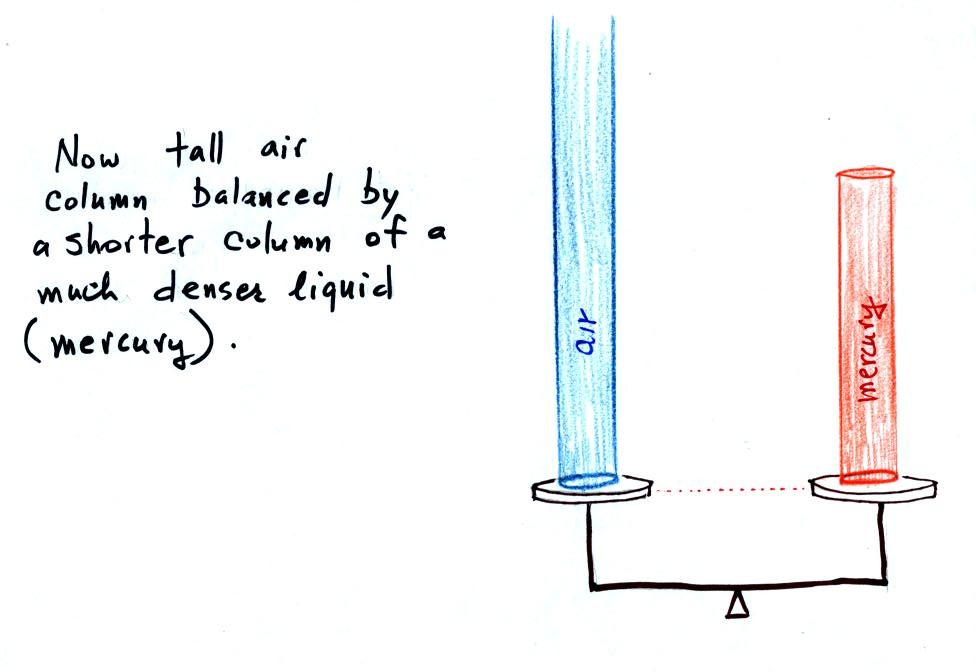
Barometers like this are usually
filled with mercury. Mercury is
a liquid. You need a liquid that can slosh back and forth in
response to changes in air pressure. Mercury is also dense which
means the barometer won't need to be as tall as if you used something
like water. A water barometer would need to be over 30 feet
tall. With mercury you will need only a 30 inch tall column to
balance the weight of the atmosphere at sea level under normal
conditions (remember the 30 inches of mercury pressure units mentioned
earlier). Mercury also has a low rate of
evaporation so you don't have much mercury gas at the top of the right
tube.

Finally here is a more conventional
barometer design.
The bowl of
mercury is usually covered in such a way that it can sense changes in
pressure but not evaporate and fill the room with poisonous mercury
vapor.