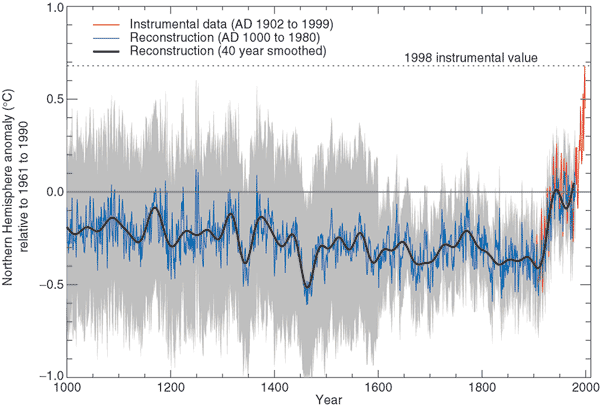
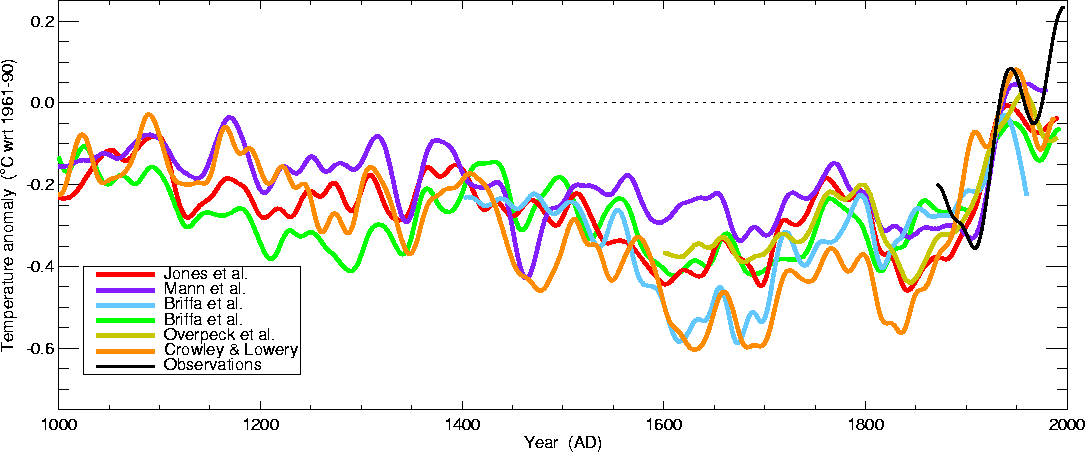
That is
where we will leave this topic for now, we've only covered a small part
of a large and ongoing debate.
SUMMARY
There is general agreement that
Atmospheric CO2 and other greenhouse
gas
concentrations are
increasing and that
The earth is warming
Not everyone agrees
on the Causes (natural or manmade) of the
warming,
how much additional warming there will be, or
on the Effects that warming will have on
weather and
climate in the years to come
We had a little bit of time left in the period so we were able to move
into the middle part of Chapter 1 and start some new, completely
different material. We will be looking
at how atmospheric characteristics such
as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we first need to understand
what pressure is and what can cause it to change.
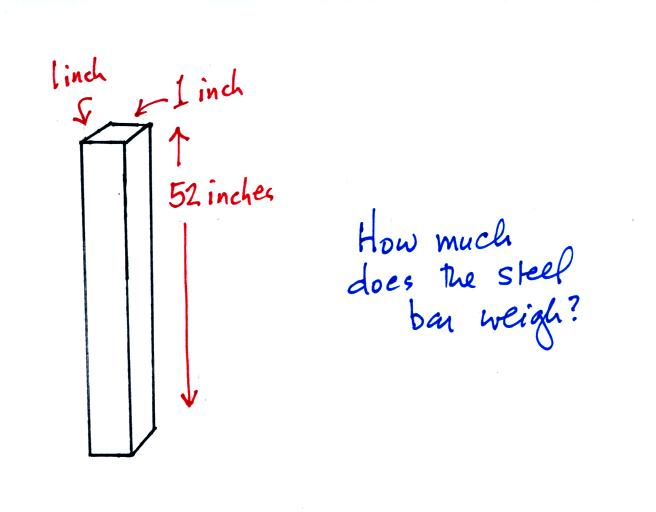
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We come back to the iron bar at the end of class.
What
follows is a little more detailed
discussion of the basic concepts of mass, weight, and density
than was covered in class.
Before we can learn about
atmospheric pressure, we
need to review
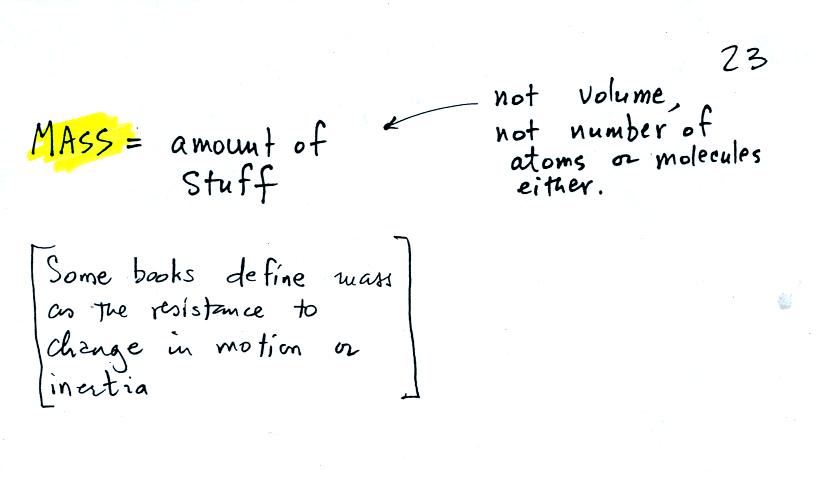
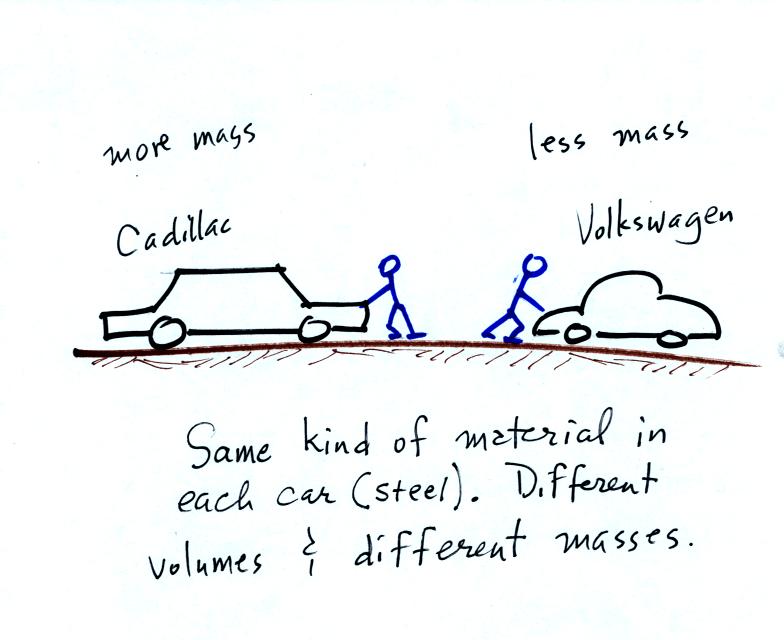
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down if it were
already moving).
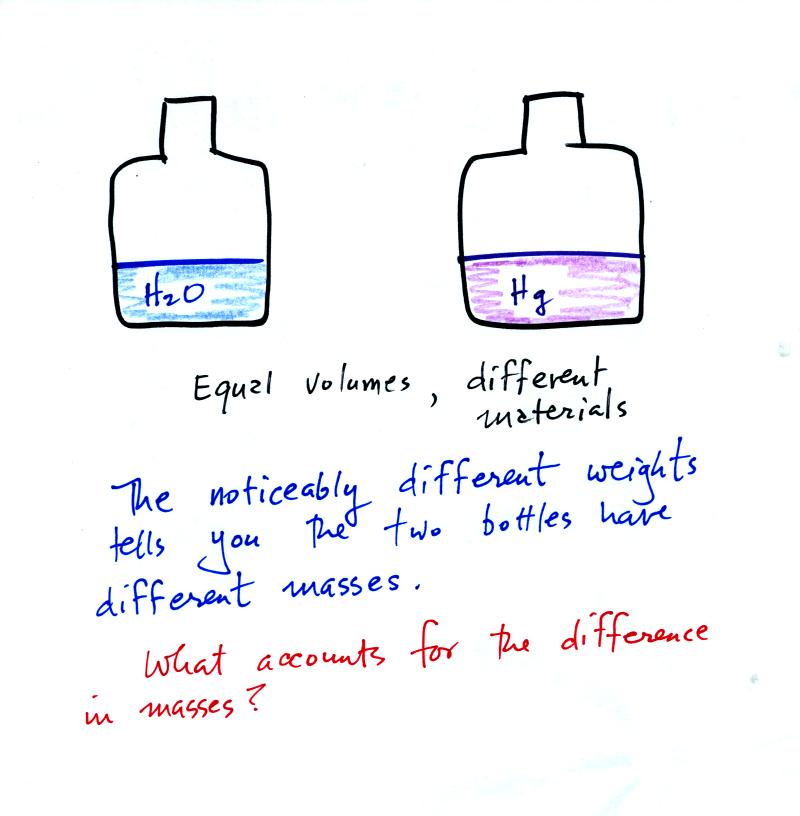
It is possible to have two objects with the
same
volume but very
different masses. The bottles of water and mercury that were
passed around class were an example (thanks for being so
careful
with the mercury).
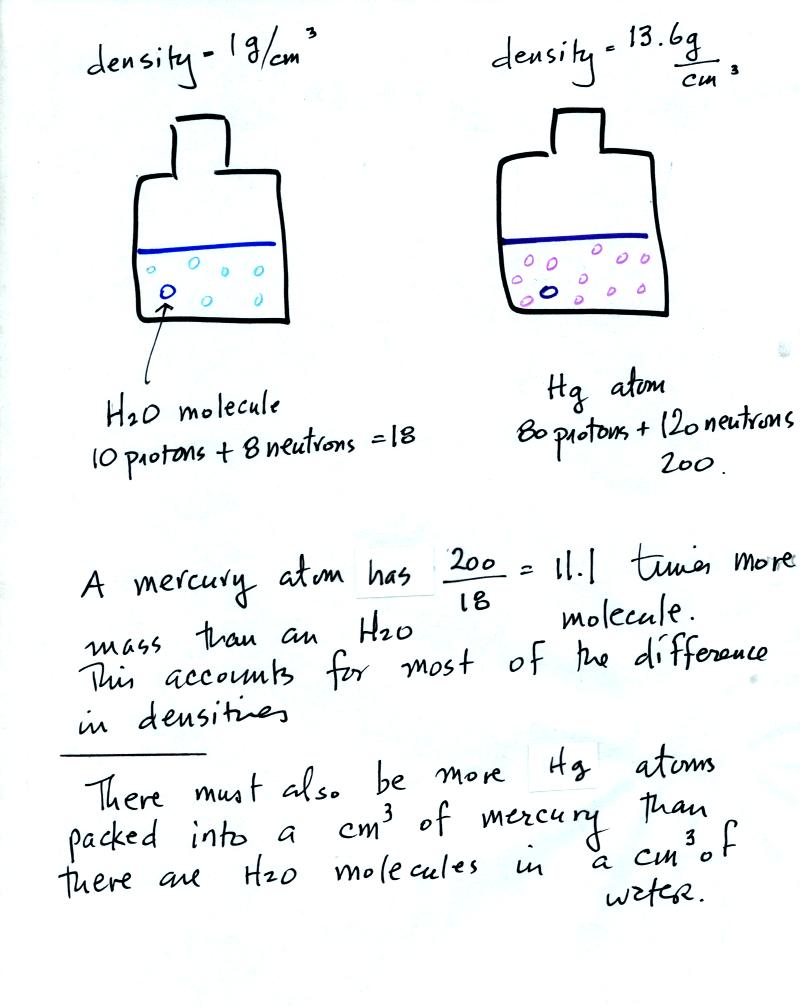
To understand why
there is such a
difference in mass and weight you need to look at the water molecules
and mercury atoms on an atomic scale.
Mercury atoms are built up of many
more protons and neutrons
than a water molecule (also more electrons but they don't have nearly
as much mass as protons and neutrons). The mercury atoms have
11.1 times as much mass as the water molecule. This doesn't quite
account for the 13.6 difference in density. Despite the fact that
they contain more protons and neutrons, the mercury atoms must also be
packed closer together than the molecules in water.
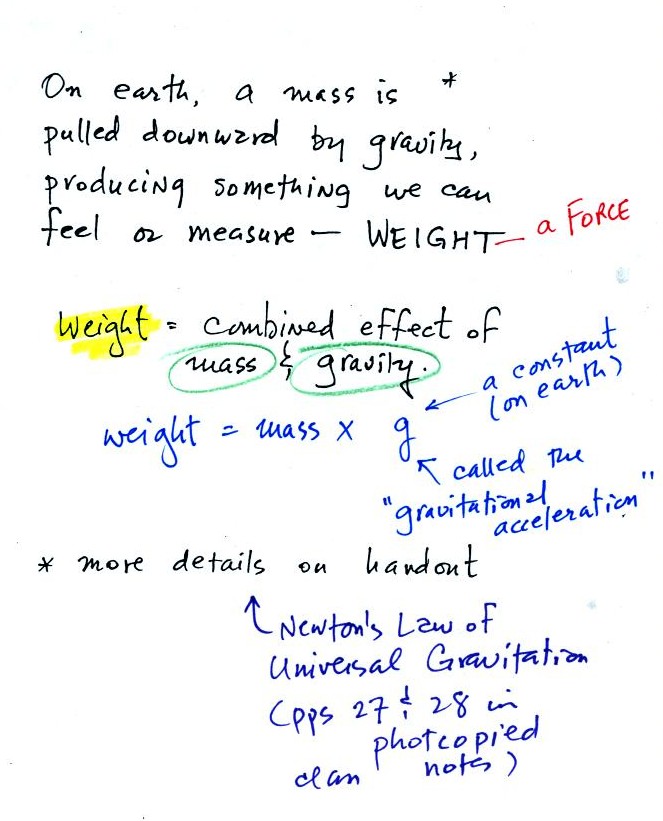
Weight
is a force and depends on
both the mass of an object and the
strength of gravity.
We tend to use weight and mass
interchangeably
because we spend all our
lives on earth where gravity never changes.
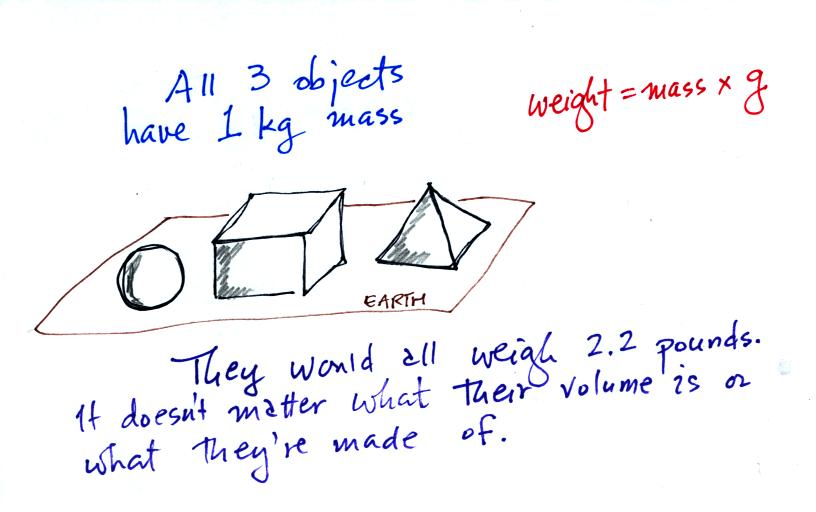
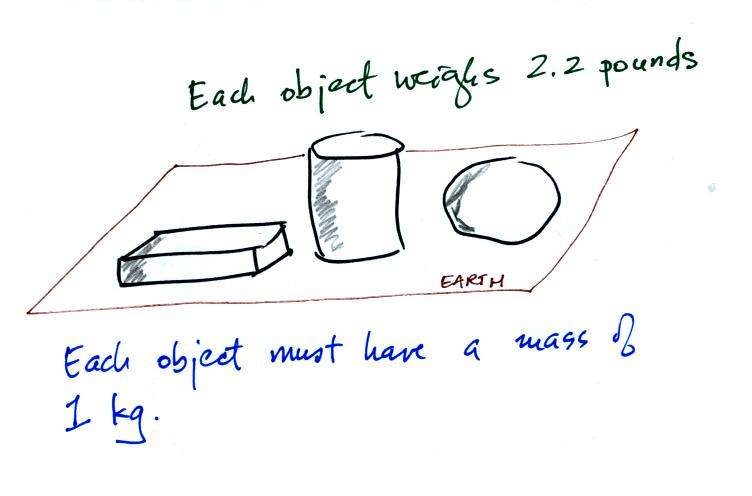
Any three objects that all have the same mass
would
necessarily have the same weight. Conversely
Three
objects with the
same weight
would also have the same mass.
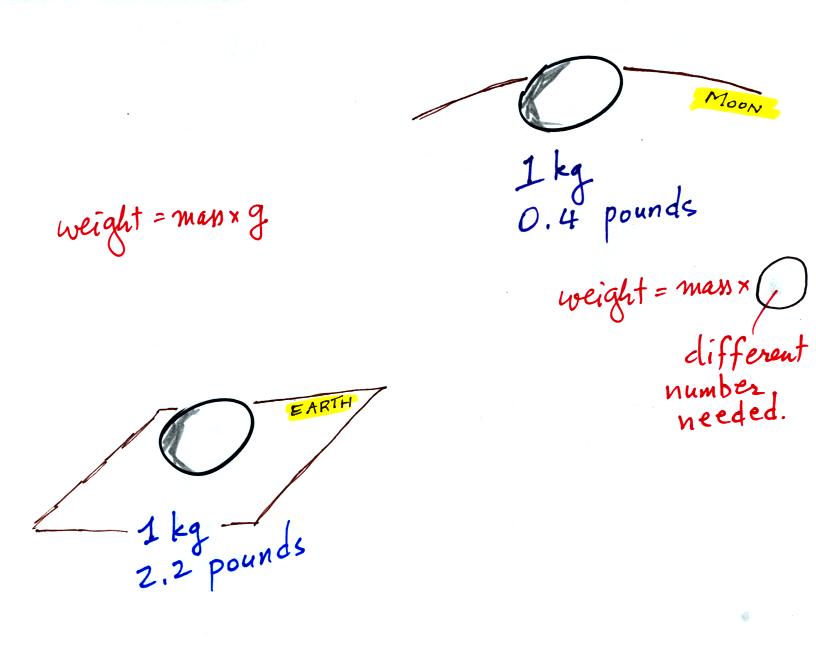
The difference between mass and weight is clearer
(perhaps) if you
compare the situation on the earth and on the moon.
If you
carry an object
from the
earth to the moon, the mass
remains the
same (its the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.
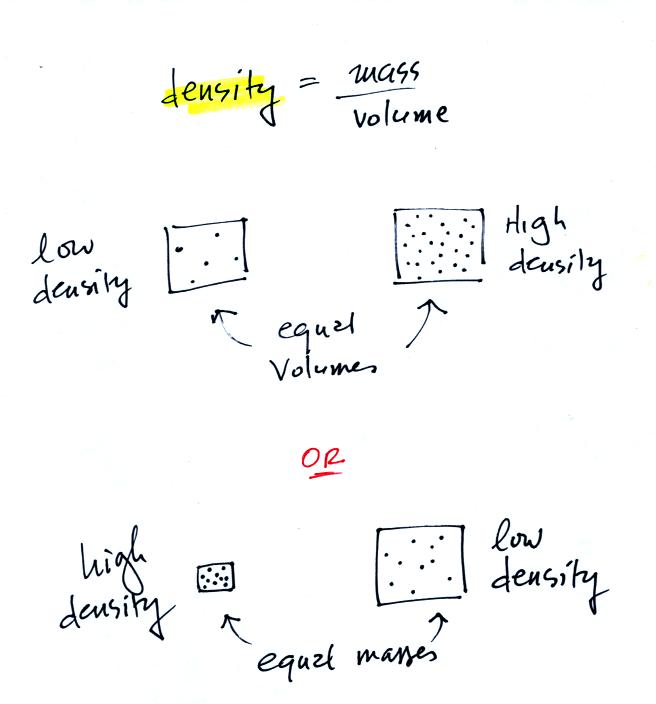
In
the first example there is more mass (more dots) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density.

The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment to prove that air has weight.
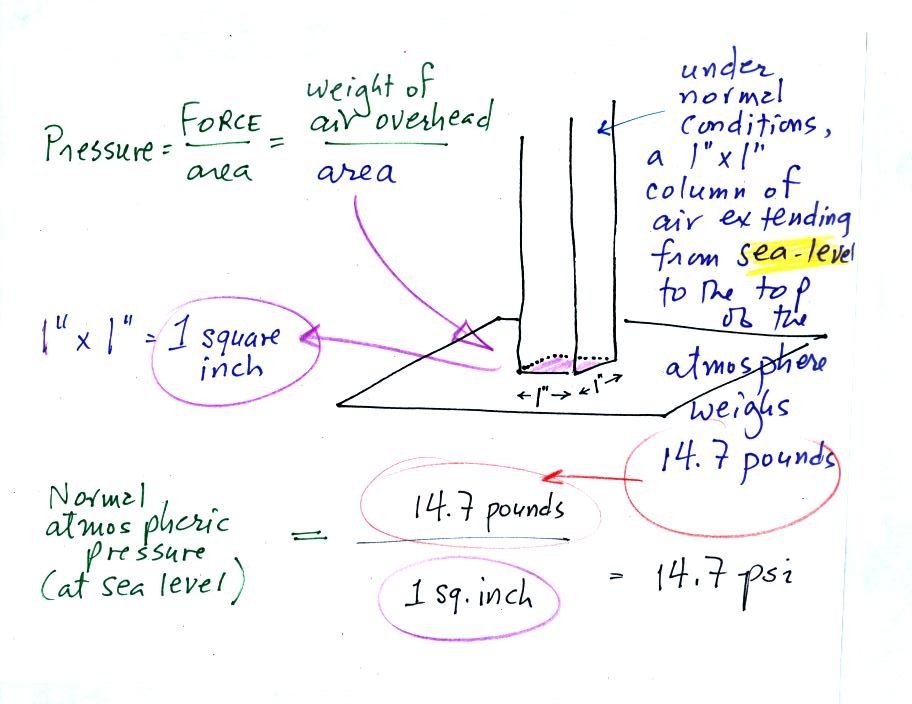
Pressure is defined as force divided by area. Air
pressure is the
weight
of the atmosphere overhead divided by the area the air is resting
on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead.
Under normal conditions a 1 inch by 1 inch column of air stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your car or bike tires with air).
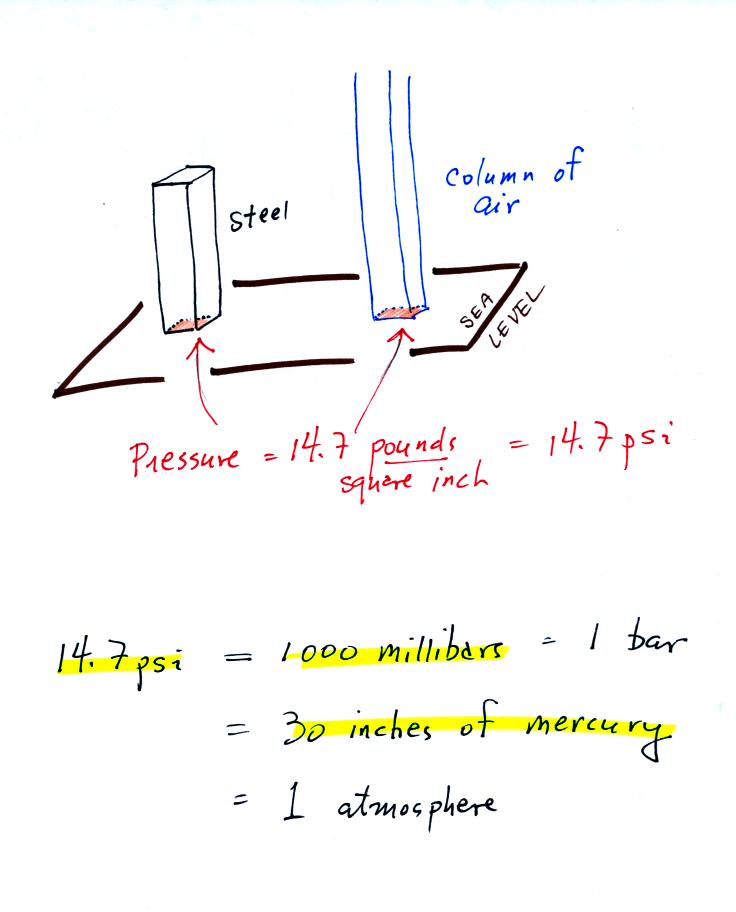
Here's the
connection between the iron bar and air pressure. The iron bar
also weighs exactly 14.7 pounds.
When it is standing on end
the bar exerts a pressure of 14.7 pounds per square inch on the ground,
the same as a 1 inch by 1 inch column of air at sea level altitude.
Some of the other commonly used pressure units are shown
above.
Typical sea level pressure is 14.7 psi or about 1000 millibars (the
units used by meterologists) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air, remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi. Peugeot 404.
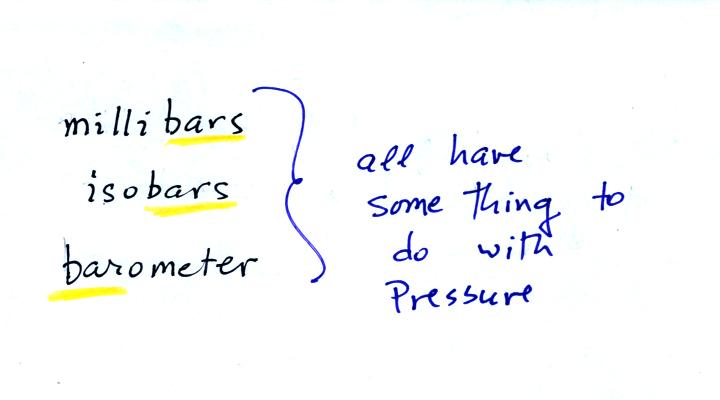
The word "bar" is used in a lot of meteorological terms: