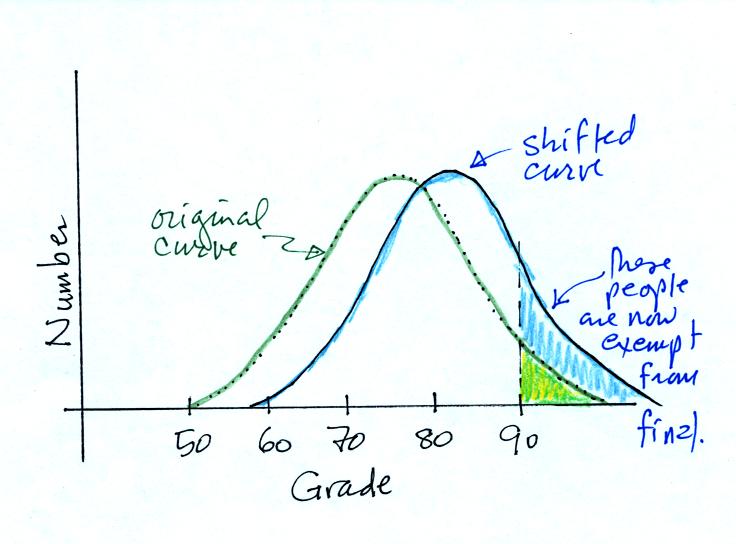
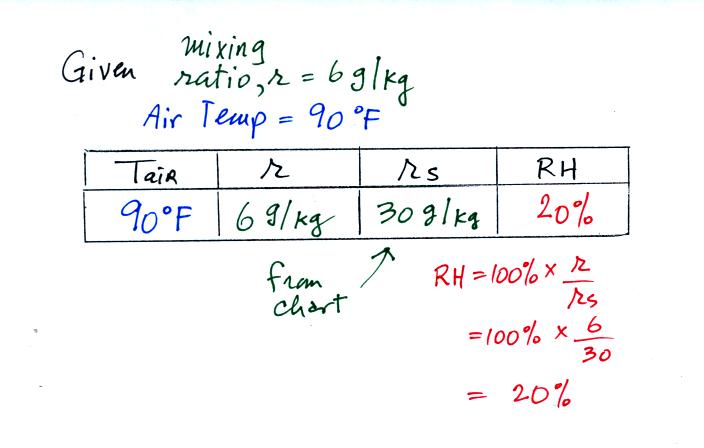
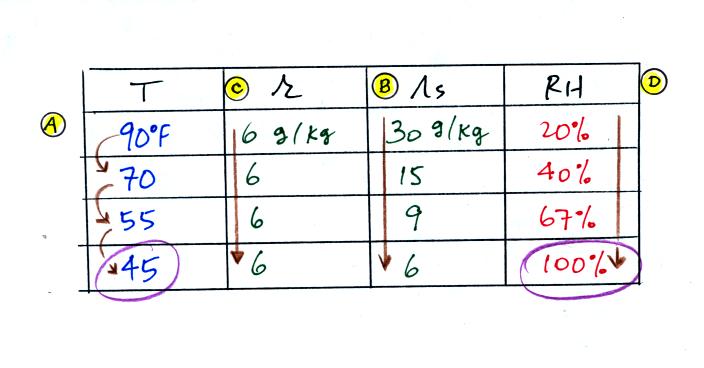
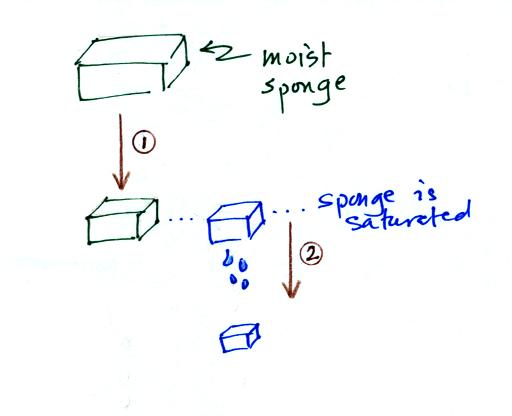
If I added 5 pts to everyones
grade,
Would the curve shift to the RIGHT or the LEFT?
Would the average grade INCREASE, DECREASE or
remain the SAME?
Would the number of people that don't have to take the final
INCREASE, DECREASE or remain the SAME?
Most everyone understood the curve would shift to the right as shown
below.
The average grade would INCREASE
and the number of people getting out of the final exam would INCREASE.