Friday Oct. 16, 2009
click here to download today's notes in
a more printer friendly mode.
A couple of songs from Dire Straits ("Sultans of Swing"
and "Walk of Life")
were played before class today.
Quiz #2 has been graded and was returned in class today. Please
check your quiz carefully for grading errors.
The Experiment #3 materials were
distributed today. I'll bring them to class again on Monday.
A new Optional Assignment (Controls of
Climate) is now available. It's due at the start of class on
Wed., Oct. 21.
A couple of new Bonus 1S1P Assignments are
now available as well.
There are a couple of loose ends to wrap up from the section
on the greenhouse effect before moving on to some new material.
You can
use the simplified picture of radiative equilibrium to understand the
effects of clouds on nighttime low and
daytime high temperatures. You'll find this discussed on pps 72a
and
72b in the Classnotes.
Here's the simplified picture of
radiative equilibrium (something
you're probably getting pretty tired of seeing). By now you
should be able to identify each of the colored arrows in the figure
above and explain what they represent.
The two pictures below show what happens at night when you remove
the
two green rays of incoming sunlight.
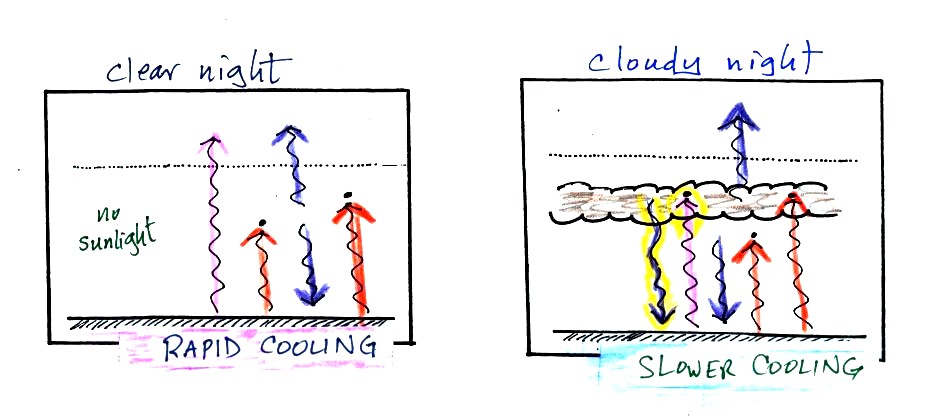
The picture on the left shows a
clear night. The ground is losing
3
arrows of energy and getting one back from the atmosphere. That's
a
net loss of 2 arrows. The ground cools rapidly and gets cold
during
the night.
A cloudy night is shown at right. Notice the effect of the
clouds.
Clouds are good absorbers
of infrared
radiation. If we could see IR light,
clouds would appear black, very different from what we are used
to (because clouds also emit IR light, if we could see IR light the
clouds might also
glow). Now none of
the IR radiation emitted by the ground passes through the atmosphere
into space. It is all absorbed either by greenhouse gases or by
the
clouds. Because the clouds and atmosphere are now absorbing 3
units of
radiation they must emit 3 units: 1 goes upward into space, the other 2
downward to the ground. There is now a net loss at the ground of
only
1 arrow.
The ground won't cool as quickly and won't get as cold on a cloudy
night as it does on a clear night. That makes for nice early
morning bicycle rides this time of the year.
The next two figures compare clear and cloudy days.
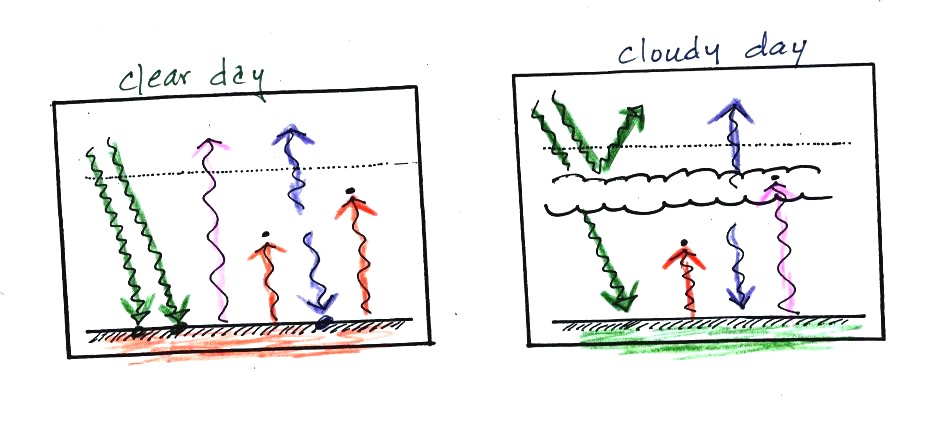
Clouds are good reflectors
of visible
light. The effect of this is to
reduce the amount of sunlight energy reaching the ground in the right
picture. With less sunlight being absorbed at the ground, the
ground
doesn't need to get as warm to be in energy balance.
It is generally cooler during the day on a cloudy day than on a
clear
day.
Clouds raise the nighttime minimum temperature and lower the
daytime
maximum temperature.
Typical daytime highs and nighttime
lows in Tucson for this
time of year. Note how the clouds reduce the daily range of
temperature.
We'll use
our simplified representation of radiative equilibrium to understand
enhancement of the greenhouse effect and global warming.

The figure (p. 72c in the
photocopied Class Notes) on the
left
shows
energy balance on the earth
without
an atmosphere (or with an atmosphere that doesn't contain greenhouse
gases). The ground achieves energy balance by emitting only 2
units of energy to balance out what it is getting from the sun.
The ground wouldn't need to be
very warm to do this.
If you add an atmosphere and greenhouse gases, the atmosphere will
begin to absorb some of the outgoing IR radiation. The atmosphere
will also begin to emit IR radiation, upward into space and downard
toward the ground. After a period of adjustment you end up with a
new energy balance. The ground is warmer and is now emitting 3
units of energy even though it is only getting 2 units from the
sun. It can do this because it gets a unit of energy from the
atmosphere.
In the right figure the concentration of greenhouse gases has
increased
even more (due to human activities). The earth would find a new
energy balance. In this case the ground would be warmer and would
be emitting 4 units of energy, but still only getting 2 units from the
sun. With more greenhouse gases, the atmosphere is now able to
absorb 3
units of the IR emitted by the ground. The atmosphere sends 2
back to the ground and 1 up into space.
The next figure shows a common misconception about the cause of
global
warming.
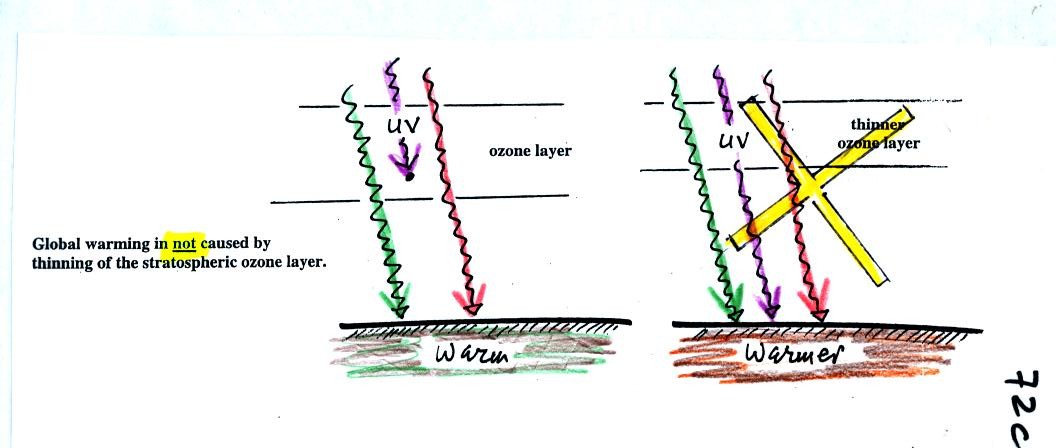
Many people know that sunlight
contains UV light and that
the ozone
absorbs much of the dangerous type of high energy radiation.
People also know that release of chemicals such as CFCs are destroying
stratospheric ozone and letting some of this UV light reach the
ground. That is all
correct.
They then conclude that it is
this additional UV energy reaching the ground that is causing the globe
to warm. This
is not correct. There isn't much UV light in sunlight in
the
first place and the small amount of additional UV light reaching the
ground won't be enough to cause global warming. It will cause
cataracts and skin cancer and those kinds of problems but not global
warming.
I spend a good part of the remainder of today's class telling you
about an awesome field experiment that I took part in several years
ago. What is the tie in with this class? A good part of the
experiment was conducted at a relatively small island near the equator
in the middle of the Pacific Ocean. Once you read the online notes on the
factors that control/determine a region's climate you will learn that
there is very little change from summer to winter in regions like this.

The photograph above appeared on the cover of the April 1994
issue of
the Bulletin of the American Meteorological Society. If you look
closely you'll notice your NATS 101 instructor (he had been given the
nickname "Wilbur" by one of the members of the group, the other bald
man's name was Orville). This photo was taken on Kapingamarangi
Atoll (shown on the map below), shortly before all the men were about
to board ship and leave Kapingamarangi. The two women (Erica at
left, Maureen in the middle) were going to remain behind and operate
all of the research equipment. The scene looks happy enough, but
"Wilbur" revealed that he had taken a liking to one of the two women
and was anything but happy.
What we were doing on Kapingamarangi? We were a small
part of a much larger field experiment. Wilbur and Orville's job
was to install the tall white lightning detector at the left edge of
the photograph. They would later travel to Rabaul (on New Britain
island) and Kavieng (New Ireland island) in Papua New Guinea and
install
two more detectors. Papua New Guinea would turn out to be a very
different place. Until recently some of the highland tribes there
practiced cannibalism. You can also get malaria in Papua
New
Guinea.
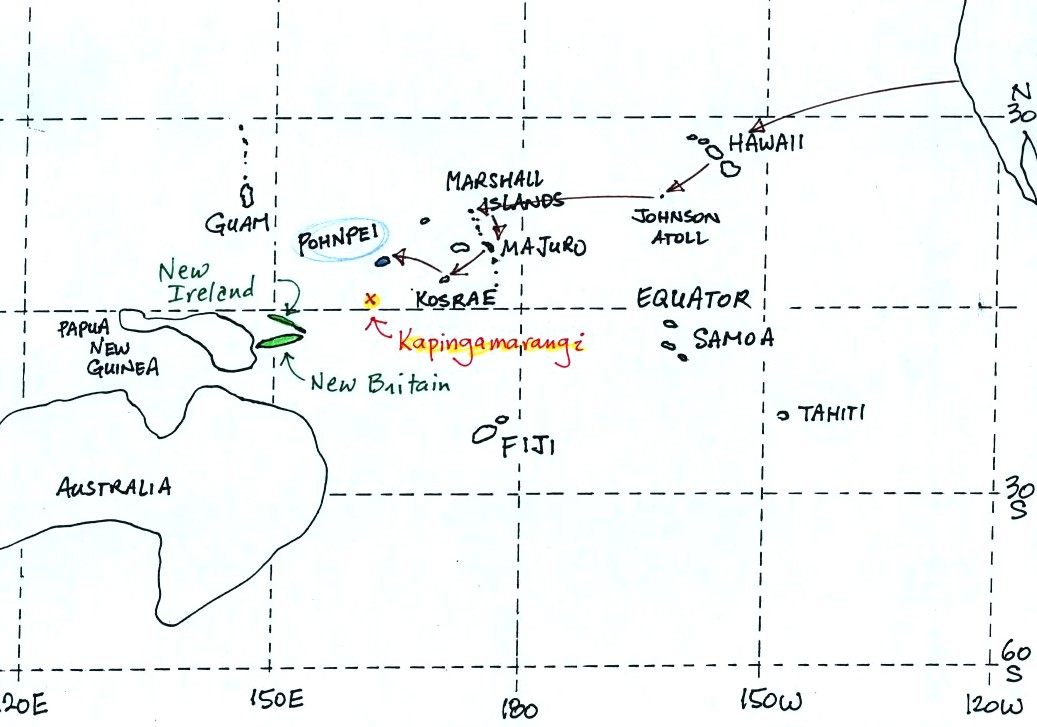
To get to Kapingamarangi you first need to fly to Pohnpei (an
island in
the Federated States of Micronesia). The route is shown
above. Then you take a cargo ship for about a 4 day sail to
Kapingamarangi. We had intended to fly to Pohnpei, set sail for
Kapinga the next day, and then spend about a month on
Kapingamarangi. The ship however was delayed 3 weeks. That
gave us plenty of time to visit the island of Pohnpei but ultimately
meant we could only spend a few days on Kapingamarangi..

Pohnpei is a fairly large island and, together with some of
the
other Micronesian islands, is a popular, world-class, snorkeling and
scuba
diving destination. Pohnpei also has a weather station that
is
operated by the US National Atmospheric and Oceanic Administration
(NOAA).
Here's a reminder of
how temperatures change during the year in Tucson.
Pohnpei is located at low latitude in the middle of the Pacific
Ocean. Both of those factors will reduce the annual range of
temperature. How
large do you think the annual range is?
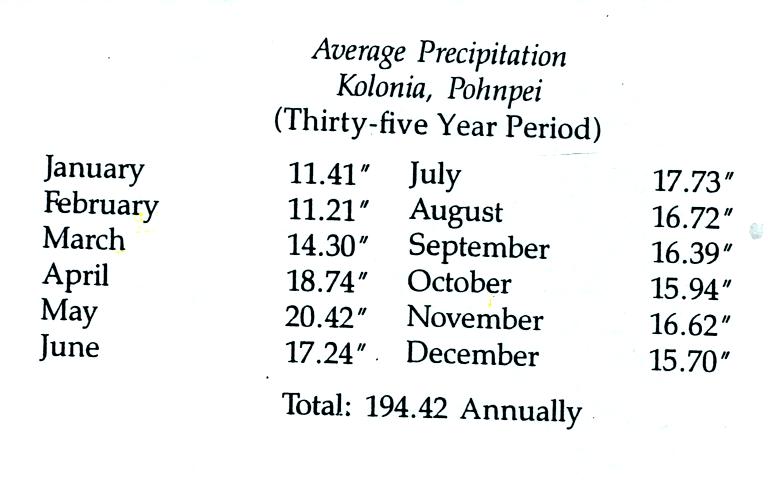
The following precipitation data show that Pohnpei is also one of
the
rainiest locations on earth
Close to 400 inches of rain may fall in the interior of
Pohnpei. The rainiest location on earth is in Hawaii with about
460 inches of
rain per year.
Pigs
are also an important part of daily life on Pohnpei, Kapingamarangi,
and the other islands in Micronesia.
The Micro Glory (shown below) sails back and forth between
Pohnpei
and Kapingamarangi about once a month. The ship carries supplies
to the people on Kapingamarangi and some other small islands.
They pay for the supplies with
pigs (the pigs are sold on Pohnpei). We shared deck space on the
Micro Glory on the trip back to Pohnpei with 20 to 30 pigs (they were
hoisted aboard in nets)
Most of the lower deck in the photo above (under the
hoists)
was occupied by pigs on the return trip. One of the pigs died on
the return trip - that was a very serious matter.
We also had a chance to sample some of the local beverages.
Drinking sakau (as it is called on Pohnpei) turns your mouth and
throat numb. It is supposed to relax you, make you sleep more
fully, and doesn't seem to have any after effects. Until fairly
recently
you could buy kava in pill form at local supermarkets. However,
because of reports that it can cause serious liver problems, that is no
longer the case. There are no reports of liver problems when
drinking kava that has been prepared in the traditional way. Here is a link to a
Wikipedia article on kava.
We never tried betelnut. Areca nuts are wrapped in betel
leaves
and chewed together with lime (lime is pretty caustic, that is one of
the reasons I didn't try betelnut). The resulting mixture is a
mild
stimulant (some people add tobacco to the mix). The most
interesting aspect, however, is that chewing betelnut colors your mouth
and teeth
bright red.
You don't
swallow betelnut, you spit it out. You see the bright red stains
on sidewalks and the ground wherever you go. Most hotels will
also have a large sign near the entrance reminding guests not to chew
betelnut inside the hotel. You can read more about betelnut here.
Try to read through the material below before class on
Monday.
The
following is an
introduction to an important new topic: humidity (moisture in the
air). This topic and the terms that we will be
learning and using can be confusing. That's the reason for this
introduction. We will be mainly be
interested in 4 variables: mixing ratio, saturation mixing ratio,
relative humidity, and dew point temperature. Our first job will
be to figure out what they are and what they're good for. Then we
see what can cause the value of each variable to change. You will
find
much of what follows on page 83 in the photocopied ClassNotes.
Mixing ratio tells you how much water vapor is actually in
the
air. Mixing ratio has units of grams of water vapor per kilogram
of dry air (the amount of water vapor in grams mixed with a
kilogram
of dry air). It is basically the same
idea as teaspoons of
sugar
mixed in a cup of tea.

The value of the mixing ratio won't change unless you add
water
vapor to or remove water vapor from the air. Warming the air
won't
change the mixing ratio. Cooling the air won't change the mixing
ratio
(unless the air is
cooled below its dew point temperature and water
vapor starts to condense). Since the mixing ratio's job is to
tell you how much water vapor is in the air, you don't want it to
change unless water vapor is actually added to or removed from the air.
Saturation mixing ratio is just an upper limit to how much
water vapor
can be found in air, the air's capacity for water
vapor. It's a
property of air, it doesn't say anything about how much water
vapor is actually in the air (that's the mixing ratio's job).
Warm air can potentially hold more
water vapor than cold air.
This variable has the same units: grams of water vapor per kilogram of
dry air. Saturation mixing ratio values for different air
temperatures are listed and graphed on p. 86 in the photocopied class
notes. You may be clicking on the words
highlighted in blue. You're probably finding that these aren't
links. One of
them is a link however, it will take you to a hidden optional
assignment that will be due at the start of the next class.
Just as is the case with water vapor in air,
there's a limit to how much sugar can be dissolved in a cup of hot
water. You can dissolve more
sugar in hot water than in cold
water.
The dependence of saturation mixing ratio on air temperature is
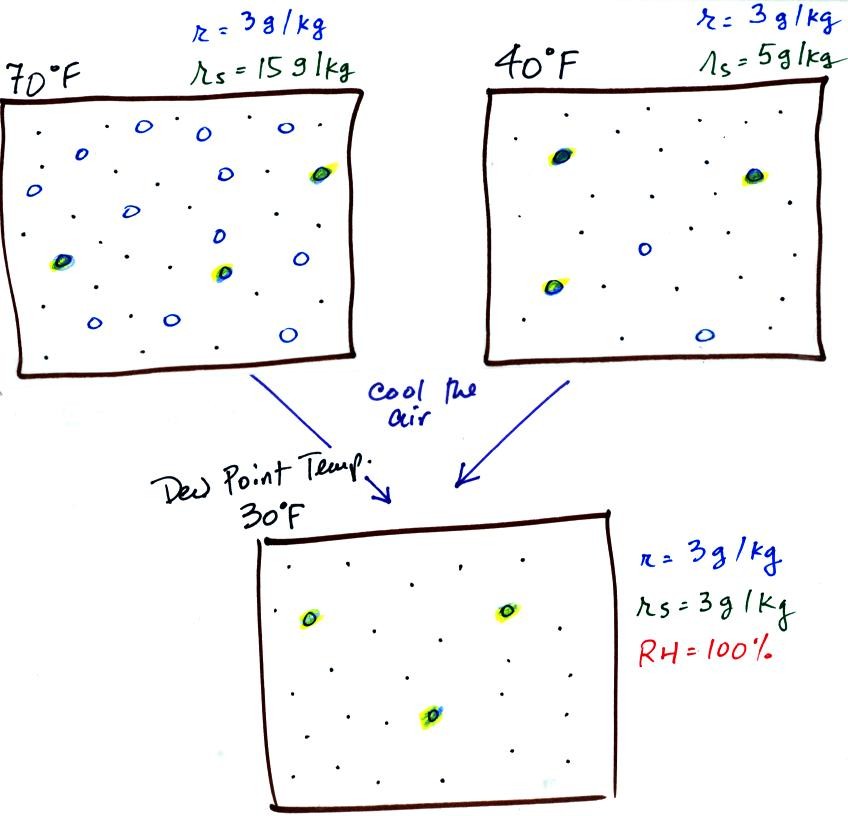
illustrated below:
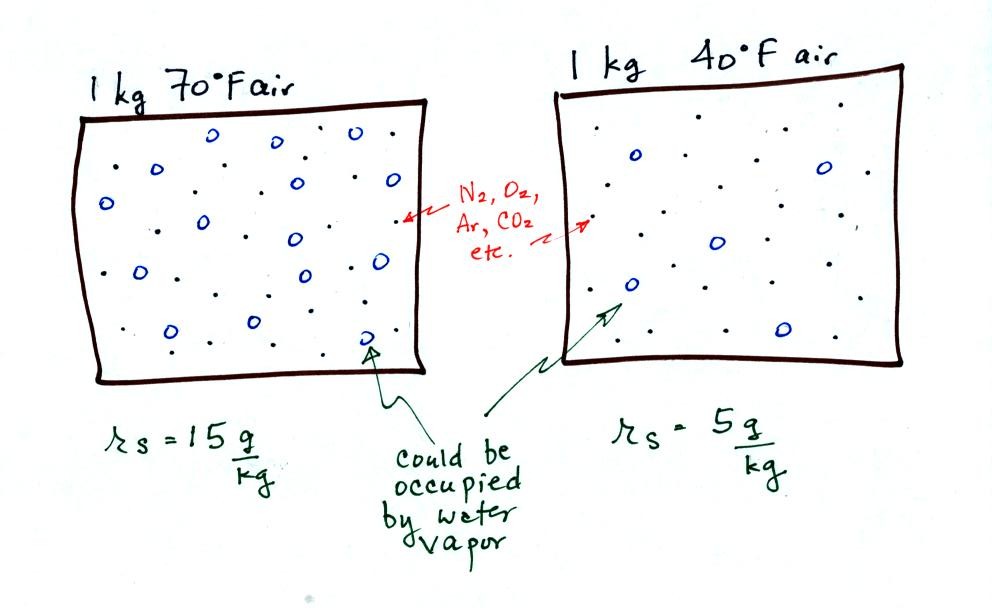
The small specks represent all of the gases in
air except
for the water
vapor. Each of the open circles represents 1 gram of water vapor
that
the air could
potentially hold. There are 15 open circles
drawn in the 1
kg of 70 F air; each 1 kg of 70 F air could hold up to 15 grams of
water vapor. The 40 F air only has 5 open circles; this
cooler
air can only hold up to 5 grams of water vapor per kilogram of dry air.

Now we have gone and actually put some water vapor
into the
volumes of
70 F and 40 F air. The same amount, 3 grams of water vapor, has
been added to each
volume of air. The mixing ratio, r, is 3 g/kg in both cases.
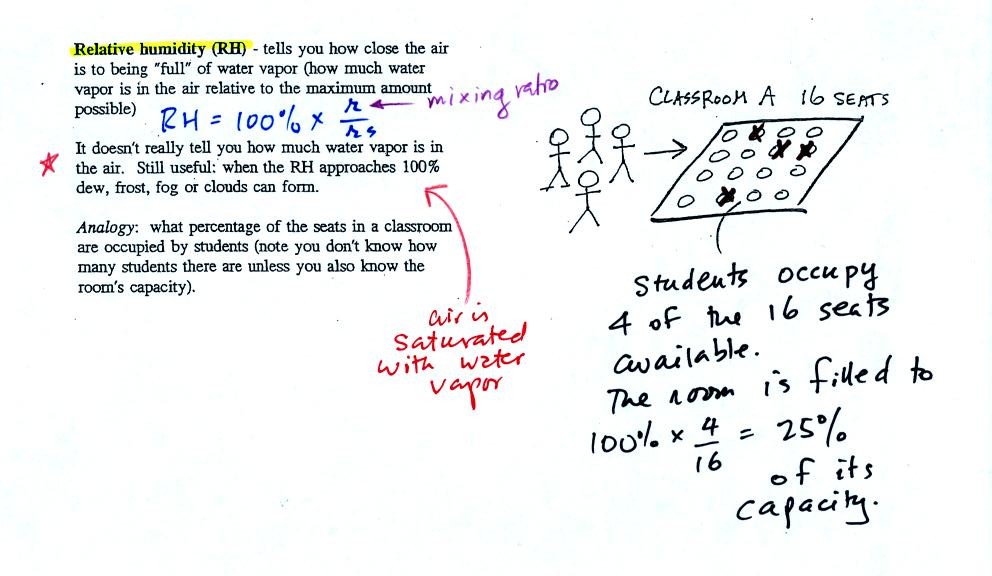
The relative
humidity is the variable most people are familiar with, it tells you
how "full" the air is with water
vapor.
In the analogy (sketched on the right hand side of p. 83 in
the photocopied notes) 4 students wander into Classroom A which has 16
empty
seats. Classroom A is filled to 25% of its capacity.
You can think of 4, the number of students, as being analogous to the
mixing ratio. The classroom capacity is analogous
to the
saturation mixing ratio. The percentage occupancy is analogous to
the relative humidity.
Instead of students and a classroom you
could think of the 70 F and 40 F air that could potentially hold 15
grams or 5 grams, respectively of water vapor. Maybe this is the
Optional Assignment I mentioned I would hide in these notes. It
will be due at the beginning of class on Mon., Mar. 23.

Here are the relative humidities of the 70 F and 40 F air
that each
contain 3 grams of water vapor. The 70 F air has a low RH because
this warm air's saturation mixing ratio is large. The RH in the
40 F is higher even though it has the same actual amount of water vapor
because the 40 F air can't hold as much
water vapor and is closer
to
being saturated.
Something important to note: RH doesn't really tell you how much water
vapor is
actually in the air. The two volumes of air above contain the
same amount of water vapor (3 grams per kilogram) but have different
relative humidities. You could just as easily have two volumes of
air with the same relative humidities but different actual amounts of
water vapor.
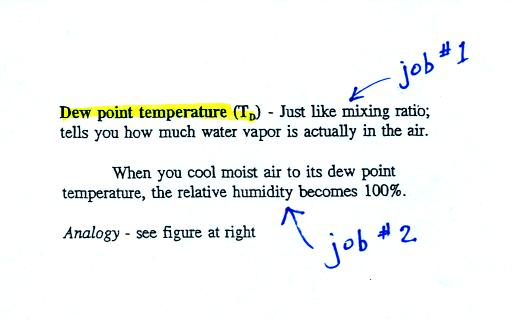
The dew point temperature has two jobs. First it gives you an
idea of
the actual amount of water vapor in the air. In this respect it
is just like the mixing ratio. If the dew point temperature is
low the air doesn't contain much water vapor. If it is high the
air contains more water vapor.
Second the dew point tells you how much you must cool the air in order
to cause the RH to increase to 100% (at which point a cloud, or dew or
frost, or fog would form).
If we cool the 70 F air or the 40 F air to 30 F we would
find that the
saturation mixing ratio would decrease to 3 grams/kilogram. Since
the air actually contains 3 g/kg, the RH of the 30 F air would become
100%. The 30 F air would be saturated, it would be filled to
capacity with water vapor. 30 F is the dew point temperature for
70 F air that contains 3 grams of water vapor per kilogram of dry
air. It is also the dew point temperature for 40 F air that
contains 3 grams of water vapor per kilogram of dry air.Because
both volumes of air had the same amount of water vapor,
they both
also have the
same dew point temperature.
Now back to our students and classrooms analogy on the
righthand
side of p. 83. The 4 students
move into classrooms of smaller and smaller capacity. The
decreasing capacity of the classrooms is analogous to the
decrease in saturation mixing ratio that occurs when you cool
air. Eventually the students move into a classroom that they just
fill to capacity. This
is analogous to cooling the air to the dew point.
If the 4 students were to move to an even smaller classroom, they
wouldn't all fit inside. The same is true of moist air. If
you cool moist air below the dew point, some of the water vapor will
condense.