This somewhat confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized. Point 5 was added
after class.
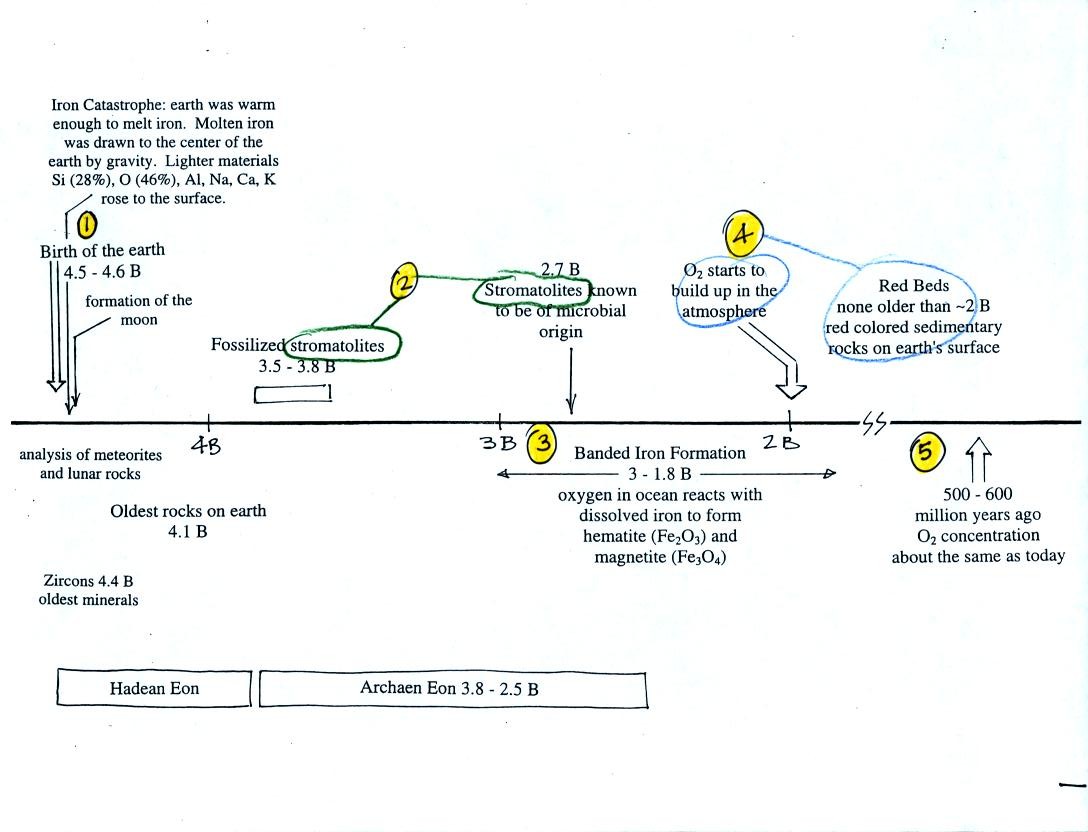
First, Point 1: the earth is thought to be between 4.5
and
4.6 billion years old.
The iron catastrophe was an important event (but wasn't
discussed in class). Circulation of liquid metal in the core of
the earth gives the earth a magnetic field. The magnetic field
deflects the solar wind around the earth. Remember the solar wind
may have swept away the earth's original atmosphere.
Stromatolites (Points 2 and 3) are column-shaped
structures made
up of layers of sedimentary rock, that are created by microorganisms
living at the top of the stromatolite (I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria
= blue green algae)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on earth. Much older (3.5 to 3.8
B years old) stromatolites presumably also produced by microbes, but
without
microbe fossils, have been found.
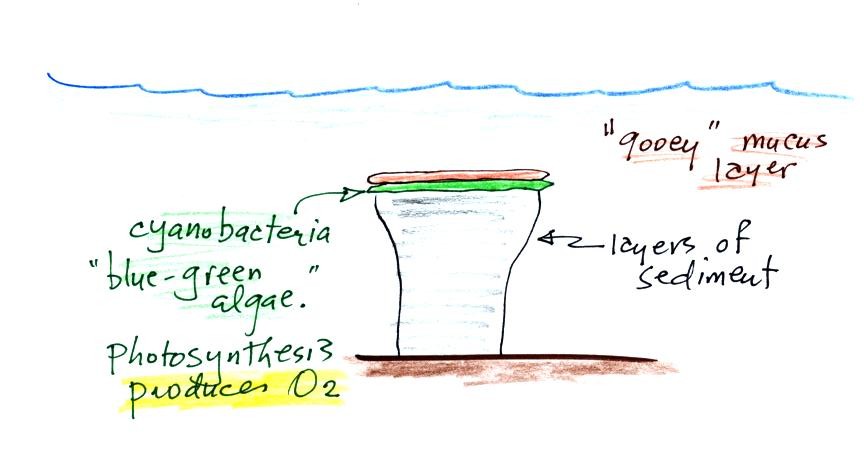
We're learning about stromatolites
because the cyanobacteria were able to produce oxygen using
photosynthesis.

Living stromatolites are found in a
few locations today. The picture above is from Coral Bay Australia, located on
the
western tip of the continent. The picture was probably taken at
low tide, the stromatolites would, I think, normally be covered with
ocean water.
Once cyanobacteria began to produce
oxygen in ocean water, the oxygen reacted with dissolved iron (iron
ions in the figure below) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed.
Periodically the oxygen production would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
might have slowed the photosynthesus). During these times of low
dissolved oxygen concentrations, layers of jasper would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded iron formation. A small polished piece of
banded iron rock (actually "tiger iron") was passed around
class. In addition to the red and black layers, the tiger
iron contains yellow layers made of fibers of quartz. The
most impressive thing about them in my opinion is
their age - they are around 3 billion years old!
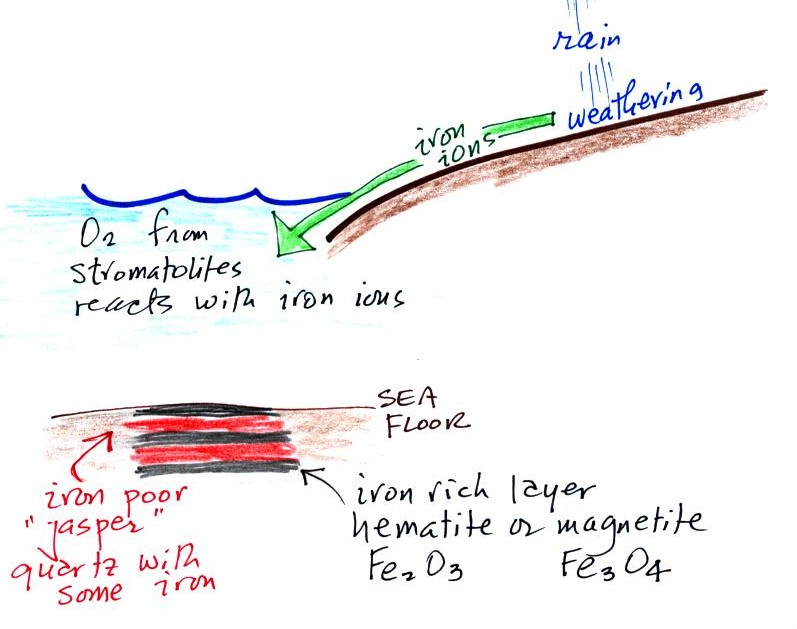
Eventually the dissolved iron in
the ocean was used up (Point 4 in the timeline figure above).
Oxygen produced by cyanobacteria no longer reacted with iron and was
free to diffuse from the ocean into the
atmosphere. Once in the air, the oxygen could react with iron in
sediments on the earth's surface. This produced red colored
(rust colored) sedimentary rock. None of these socalled red beds
are older than
about 2 B years old. Thus it appears that a real buildup up
oxygen began around 2 B years ago. Oxygen concentrations reached levels
that are about the same as today around 500 to 600 years ago (Point 5
in the figure).
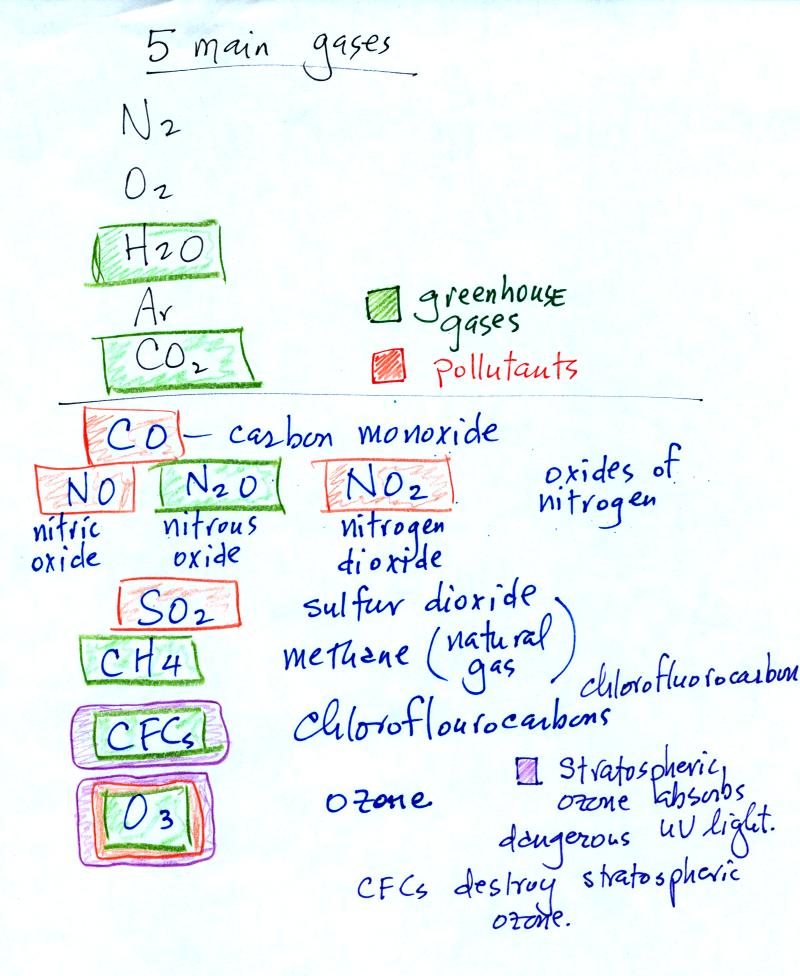
We listed
the 5 most abundant gases in the atmosphere in class on Tuesday.
Several more important trace gases were added to the
list in
class today. Trace gases are gases found in low
concentrations. Low concentrations doesn't mean they aren't
important, however.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing gas),
chlorofluorocarbons, and ozone are all greenhouse gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic more next week and learn more about how the
greenhouse effect actually works later in the course.
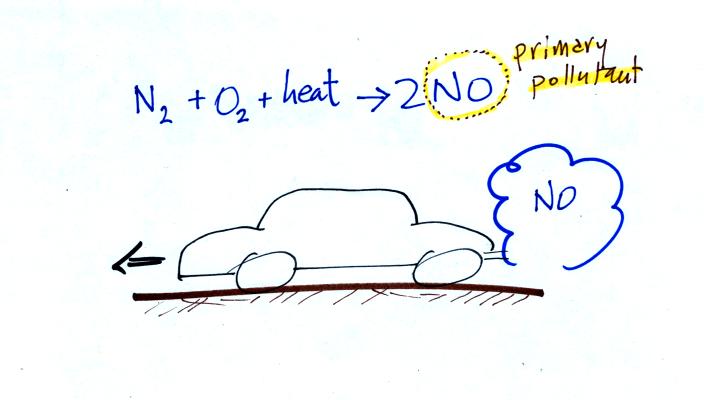
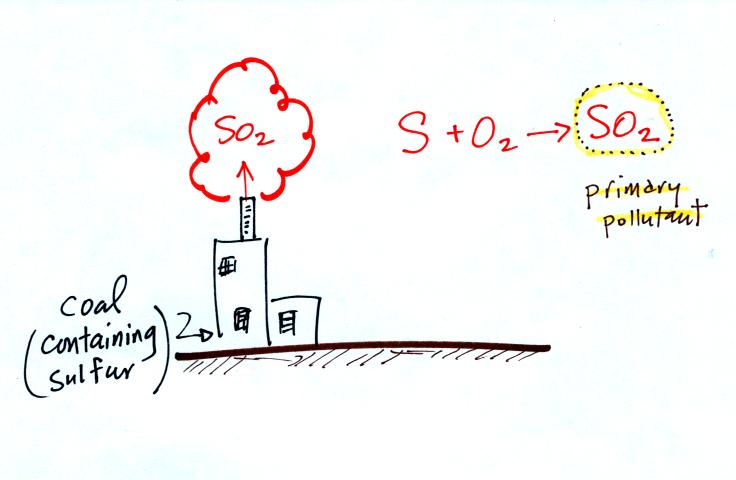
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants.
Be careful with ozone:
(i) Ozone in the
stratosphere (a layer of the atmosphere between 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
We'll start discussing air
pollution in class today and finish up next week. Air
Pollution is a serious health hazard in the US and around the
world. The following statistics were shown in class.
Click here
to download a copy of this information.

Keep in mind that many of these
numbers are difficult to measure
and some may contain a great deal of uncertainty. The row that is
highlighted, toxic agents, contains estimates of deaths caused by
indoor and outdoor air pollution, water pollution, and exposure to
materials such as asbestos and lead both in the home and at the work
place. It is estimated that 60% of the deaths are due to exposure
to particulate matter, something that we will examine in a little more
detail next week.

Air pollution is a serious hazard
worldwide. Interestingly indoor air pollution is, in many places,
a more serious threat than outdoor air pollution.
The Blacksmith
Institute has listed the Top 10 polluted places in the world in a
2007 report. The report has received a lot of worldwide
attention. If you go to this
address, you can view the report online or download and print a
copy of the report. This is just in case you are interested, this wasn't discussed in class.
It's always nice to be able to see
what we're covering in class.
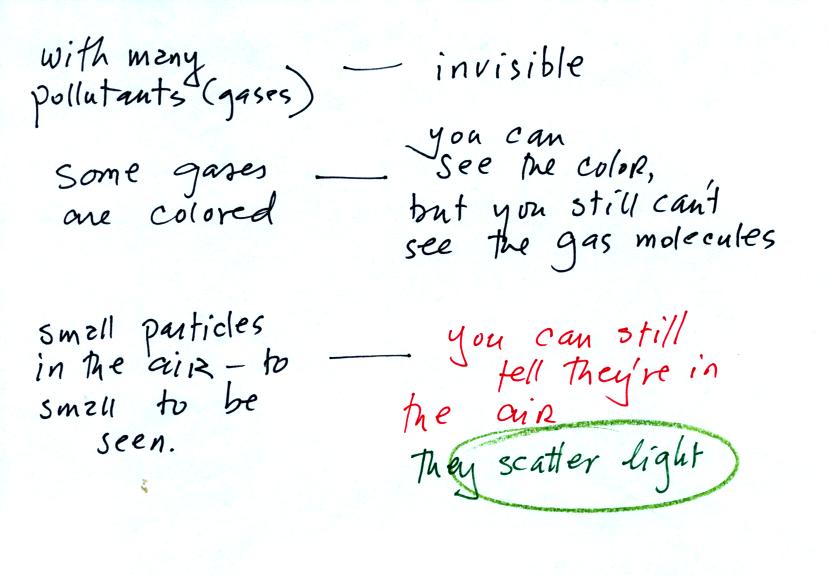
Many of the gases in the atmosphere are invisible. Some are
colored. We'll make some nitrogen dioxide at the end of the class
today. Nitrogen dioxide has a reddish brown color and is
visible. We are able to see clouds, smog, fog, and haze even
though the particles that make them up are too small to be seen.
We can see them because they scatter light. The blue color of the
sky is produced by scattering of sunlight by air molecules.
We did a short
demonstration to explain and illustrate light scattering.
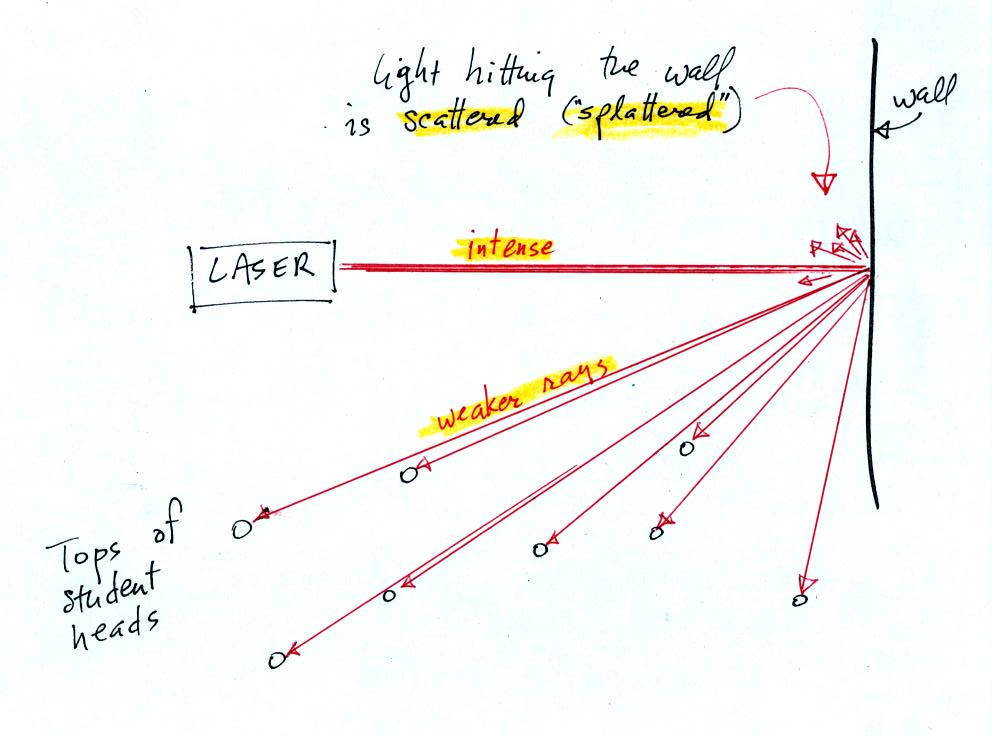
In the first part of the demonstration a narrow beam of intense red
laser light was shined from one side of the classroom to the
other.
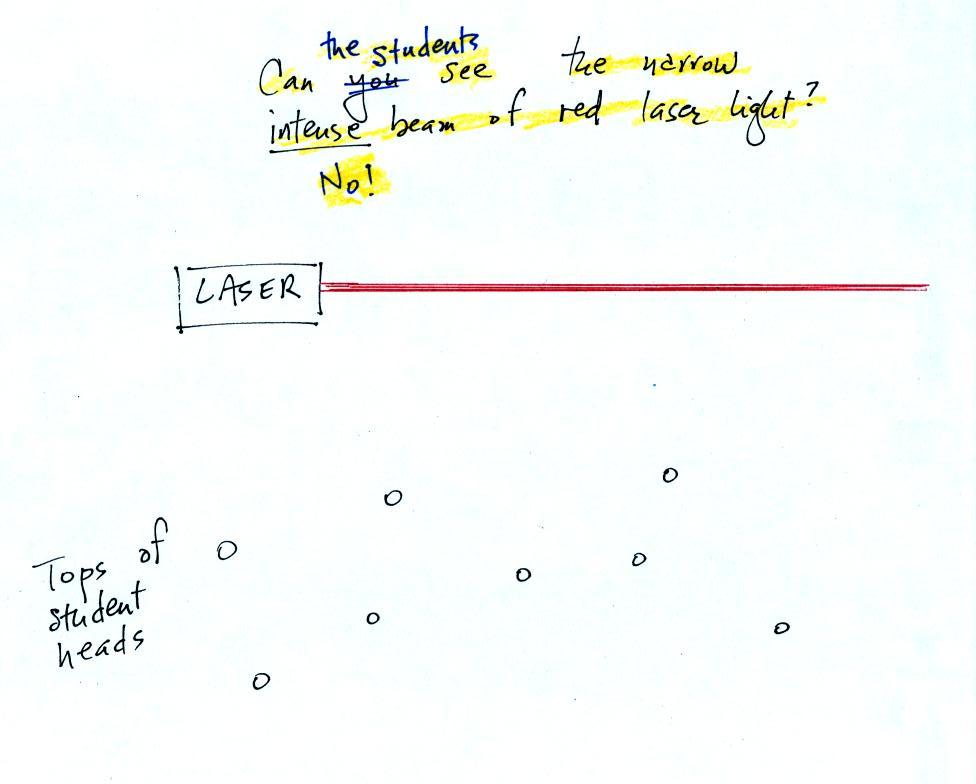
Students were able to see a bright red spot where the laser beam struck
the wall.
This is because when the intense beam of laser light
hits the wall it
is scattered (splattered is a more descriptive term). Weaker rays
of light are sent out in all directions. There is a ray of light
sent in the direction of every student in the class. They see the
light because they are looking back in the direction the ray came
from. It is safe to look at this light because the rays are
weaker than the initial beam.
Next we clapped some erasers together so that some small
particles of chalk dust fell into the laser beam.
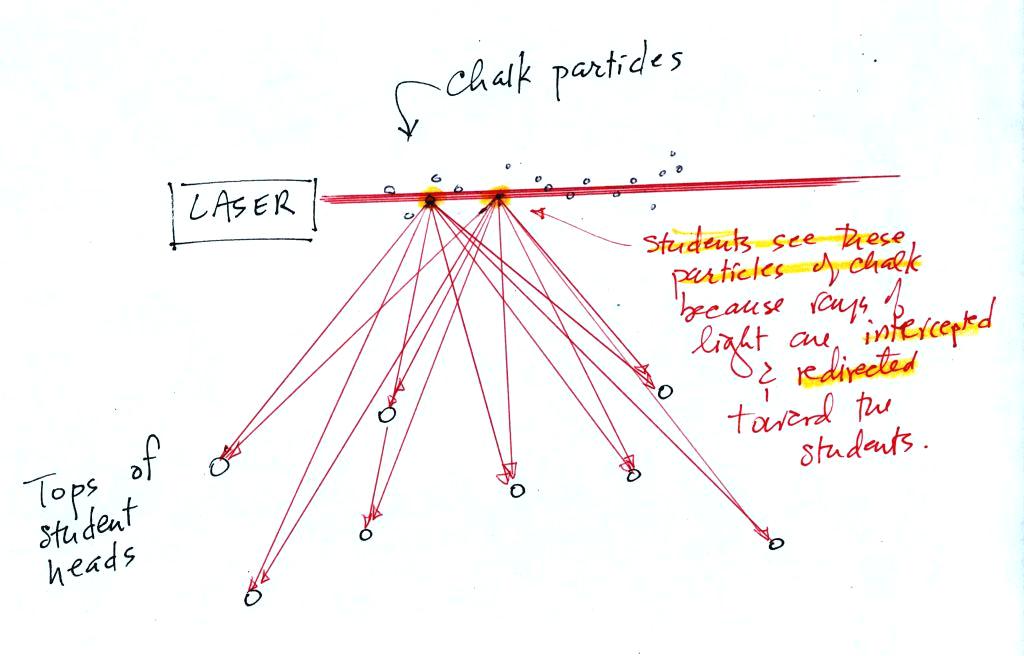
Now instead of a single spot on the wall, students
saws lots of
points of light coming from different positions along the laser
beam. Each of these points of light was a particle of chalk, and
each piece of chalk dust was intercepting laser light and sending light
in all directions. Each student saw a ray of light coming from
each of the chalk particles.
We use chalk because it is white, it will scatter rather
than absorb visible light. What would you have seen if black
particles
of soot had been dropped into the laser beam?
In the last part of the demonstration we made a cloud by
pouring some
liquid nitrogen into a cup of water. The numerous little water
droplets made very good scatterers.

A comment not mentioned in
class. Air molecules are able
to scatter light too, just like cloud droplets. Air molecules are
much smaller than cloud droplets and don't scatter much light.
That's why you were able to see light being scattered by air before we
put chalk particles or cloud droplets into the beam. Outdoors you
are able to see sunlight (much more intense than the laser beam used in
the class demonstration) scattered by air molecules. Sunlight is
white and is made up of violet, blue, green, yellow, orange, and red
light. Air molecules have an unusual property: they scatter the
shorter wavelengths (violet, blue, green) much more readily than the
longer wavelength colors in sunlight (yellow, orange, and red).
When you look away from the sun and look at the sky, the blue color
that you see are the shorter wavelengths in sunlight that are being
scattered by air molecules. We'll come back to this later in the
semester.
We started to learn a little bit about carbon monoxide before moving to
the last demonstration of the day. We
will be talking about carbon monoxide found both outdoors
(where it rarely would reach fatal concentrations) and indoors (where
it can be deadly). The
following is found on the top of p. 7 in the photocopied ClassNotes.
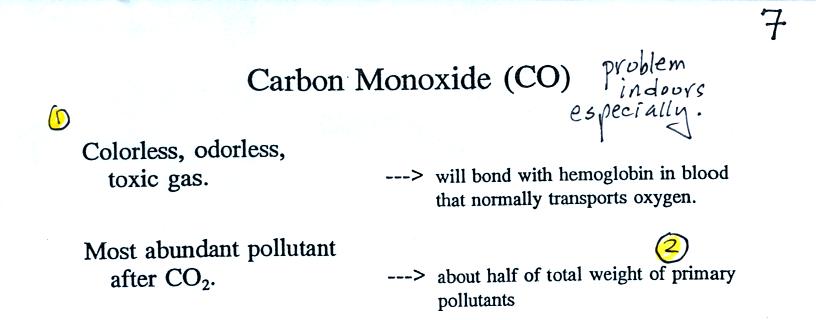
Carbon monoxide is insidious, you can't smell
it or see it
and it can kill you. Once inhaled, carbon monoxide molecules bond
strongly
to the hemoglobin
molecules in
blood and interfere with the transport of oxygen throughout your
body.
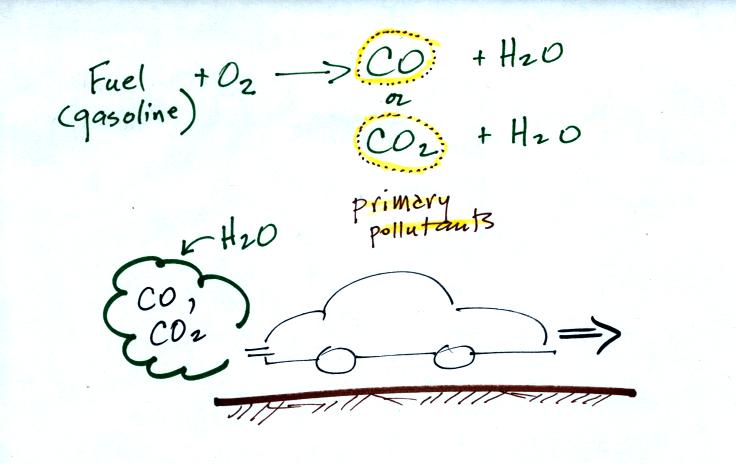
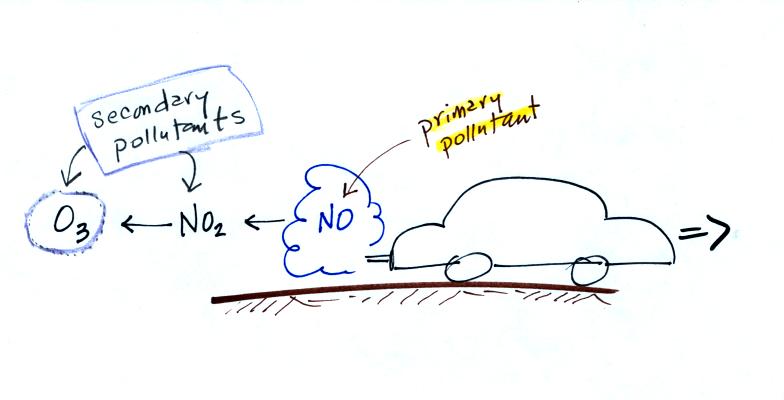
CO is a primary pollutant (Point 2 above). That means it goes
directly from a source into the air, CO is
emitted directly from an automobile tailpipe into the atmosphere for
example. The difference between
primary and secondary pollutants is probably explained
best in a series of pictures.