Thursday Oct. 22, 2009
click here to download today's notes
in a more printer friendly format.
Some zydeco music from the Best of Beausoleil CD before class this
morning.
The Experiment #2 reports have been graded
and were returned in class today. You are allowed to revise your
report; revisions are due on or before Thu., Nov. 5. Please
return your original report with your revised report.
The Experiment #4 materials were
distributed in class today. Experiment #4 reports are due on
Tue., Nov. 10.
A new Humidity
Optional Assignment was distributed in class. The assignment
is due next Thursday, Oct. 29. There was also an In-class
Optional Assignment today. You'll find the questions below
embedded in the online notes. You may turn in answers to the
questions if you wish at the start of class next Tuesday.
We'll work
through 4 humidity example problems today.
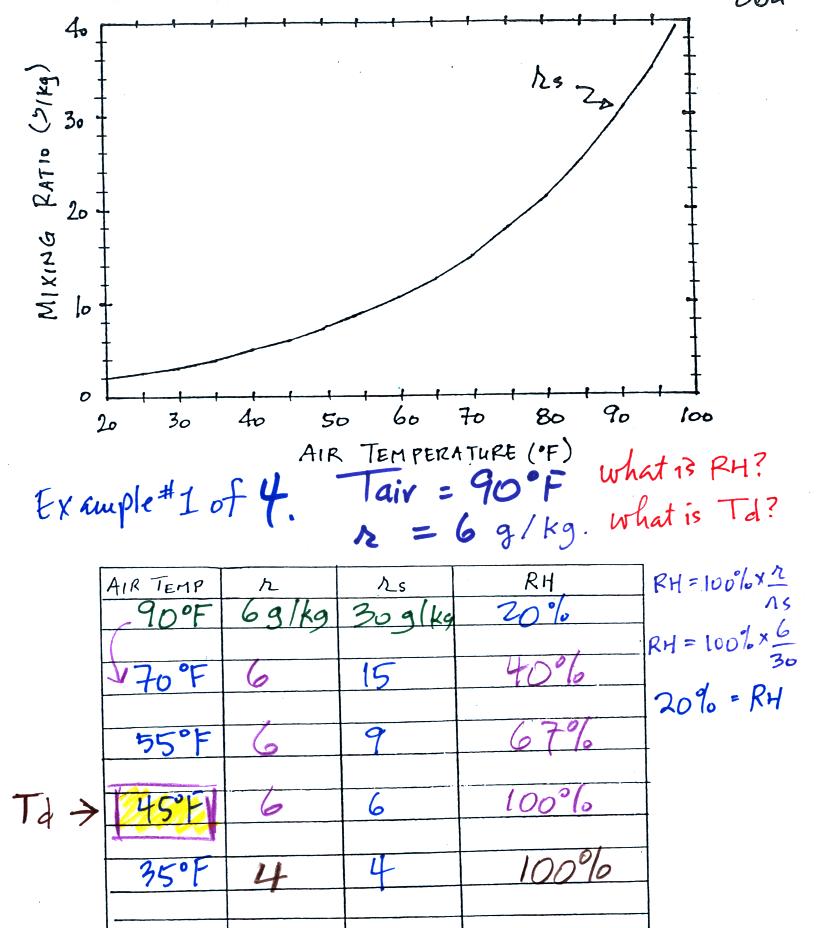
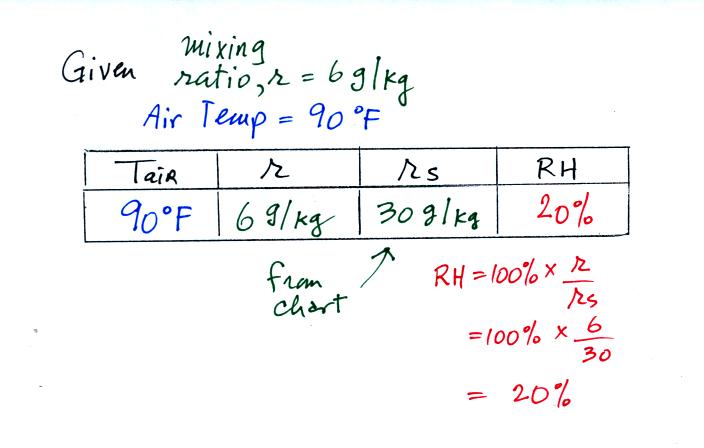
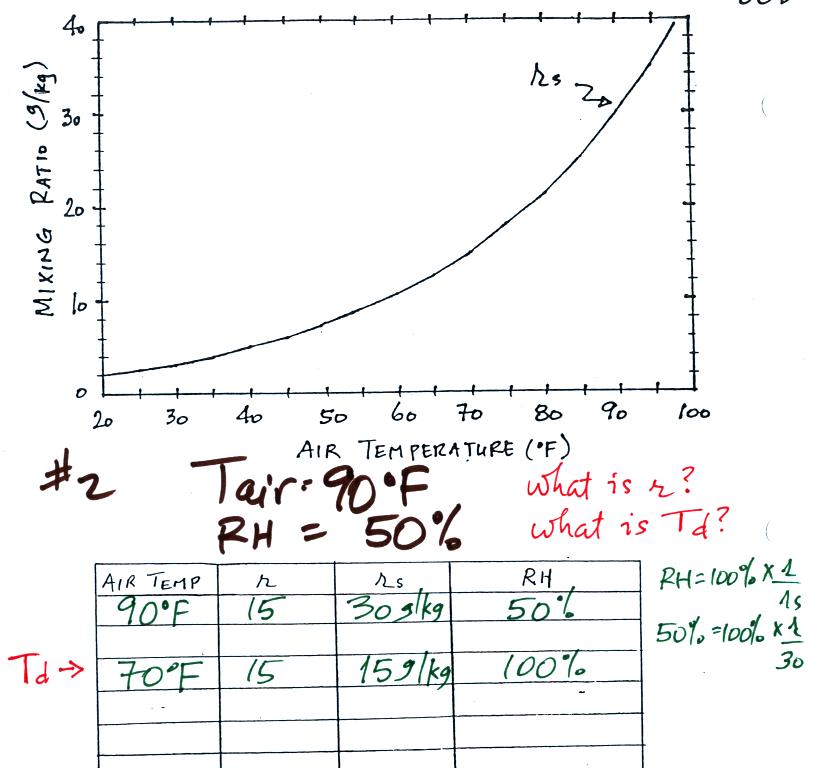
Example 1
We were given an air temperature of 90 F and a mixing ratio (r) of 6 g/kg.
We're supposed to find the relative humidity (RH) and
the dew point temperature (Td). You might have a hard
time
understanding this if
you're seeing it for
the first
time. The series of steps that we followed are retraced
below:
We start by entering the data we were given in the
table. Once
you know the air's temperature you can look up the saturation mixing
ratio value; it is 30 g/kg for 90 F air. 90 F air could
potentially hold 30 grams of water vapor per kilogram of dry air (it
actually contains 6 grams per kilogram in this example). A table
of
saturation mixing ratio values can be found on p. 86 in the ClassNotes.
Once you know mixing ratio and saturation mixing ratio you can
calculate the relative humidity (you divide the mixing ratio by the
saturation mixing ratio, 6/30, and multiply the result by 100%).
You ought to be able to work out the ratio 6/30 in your head (6/30 =
1/5 = 0.2). The RH is 20%.
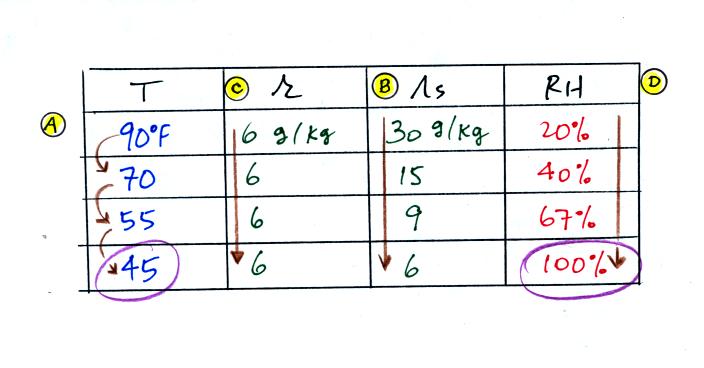
The numbers we just figured out are shown on the top line
above.
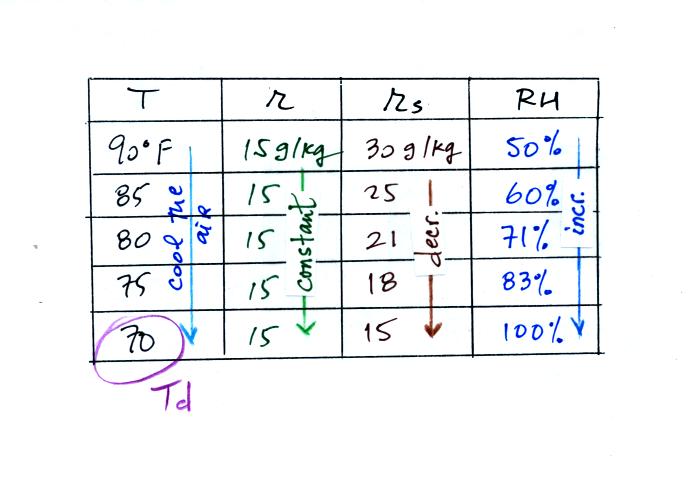
(A) We imagined cooling the air from 90F to 70F, then to 55F, and
finally to 45F. Pay attention to what is happening to the mixing
ratio, saturation mixing ratio, and relative humidity as the air cools.
(B) At each step we looked up the saturation mixing ratio and entered
it on the chart. Note that the saturation mixing ratio values
decrease as the air is
cooling.
(C) The mixing
ratio doesn't
change as we cool the air. The only
thing that changes r is adding or removing water vapor and we aren't
doing either.
(D) Note how the relative
humidity is increasing as we cool
the
air. The actual amount of water vapor in the air isn't changing,
it is just the air's capacity that is decreasing.
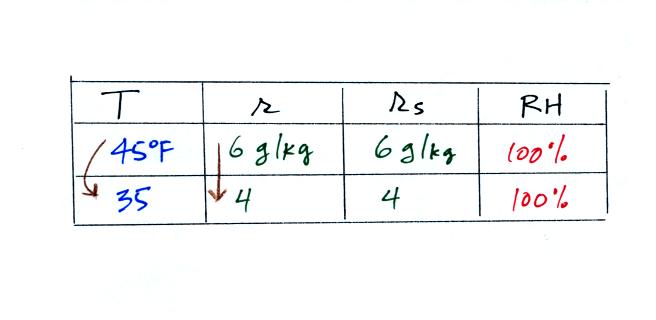
Finally at 45 F the RH becomes 100%. You have cooled the air
until it has become
saturated. This is a special point, this is the dew
point
temperature.
What would happen if we cooled the air
further still, below the dew
point temperature?
35 F air can't hold the 6 grams of water vapor
that 45 F air can. You can only "fit" 4 grams of water vapor into
the 35 F air. The remaining 2 grams of water vapor would condense
and form water. If
this happened at ground level the ground would get wet with dew.
If it happens above the ground, the water vapor condenses onto small
particles in the air and forms fog or a cloud. Because water
vapor is being taken out of the air (and being turned into water), the
mixing
ratio will now decrease from 6 to 4.
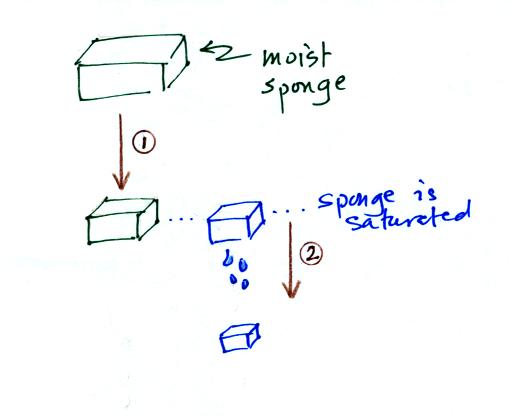
In many ways cooling moist air is liking squeezing a
moist sponge
Squeezing the
sponge and reducing its volume is like cooling moist air and reducing
the saturation mixing ratio. (1) At first when you sqeeze the
sponge
nothing happens, no water drips out. Eventually you get to a
point where the sponge is saturated. This is like reaching the
dew point. (2) If you squeeze the sponge any further (or cool air
below
the dew point) water will begin to drip out of the sponge (water vapor
will condense from the air).
Example 2
The work that we did in class is shown above. Given an air
temperature
of 90
F and a relative humidity of 50% you are supposed to figure out the
mixing ratio (15 g/kg) and the dew point temperature (70 F). The
problem is worked out in detail below:
First you fill in the air temperature and the RH data that
you are
given.
(A) since you know the air's temperature you can look up the
saturation mixing ratio (30 g/kg).
(B) Then you might be able to figure out the mixing ratio in your
head. Air that is filled to 50% of its capacity could hold up to
30 g/kg. Half of 30 is 15, that is the mixing ratio. Or you
can substitute into
the relative humidity formula and solve for the mixing ratio.
Finally you imagine cooling the air. The
saturation mixing ratio decreases, the mixing ratio stays constant,
and the relative humidity increases. In this example the RH
reached 100% when the air had cooled to 70 F. That is the dew
point temperature.
We can use
results from humidity problems #1 and #2 worked in class on Monday to
learn a useful rule.
In the first
example the difference between the air and dew point
temperatures was large (45 F) and the RH was low (20%).
In the 2nd problem the difference between the air and dew point
temperatures was
smaller (20 F) and the RH was higher (50%). The easiest way to
remember
this
rule is to remember the case where there is no difference between the
air and dew
point temperatures. The RH then would be 100%.
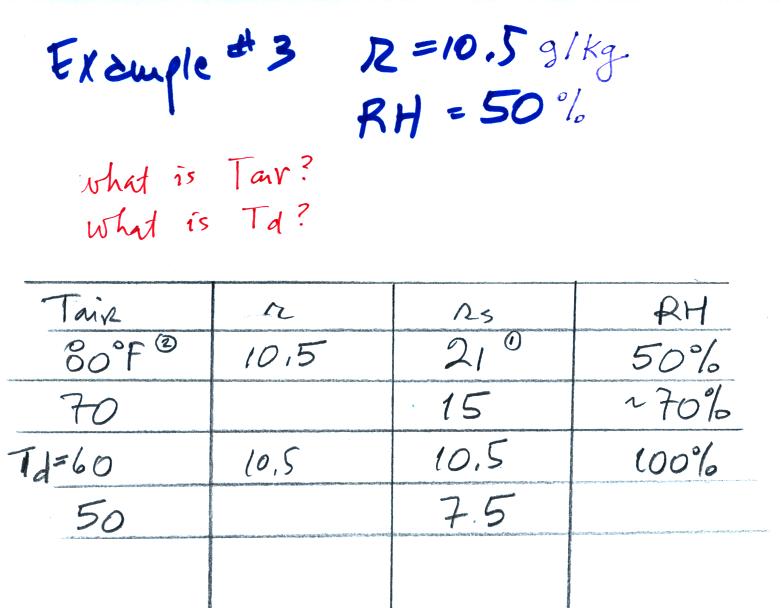
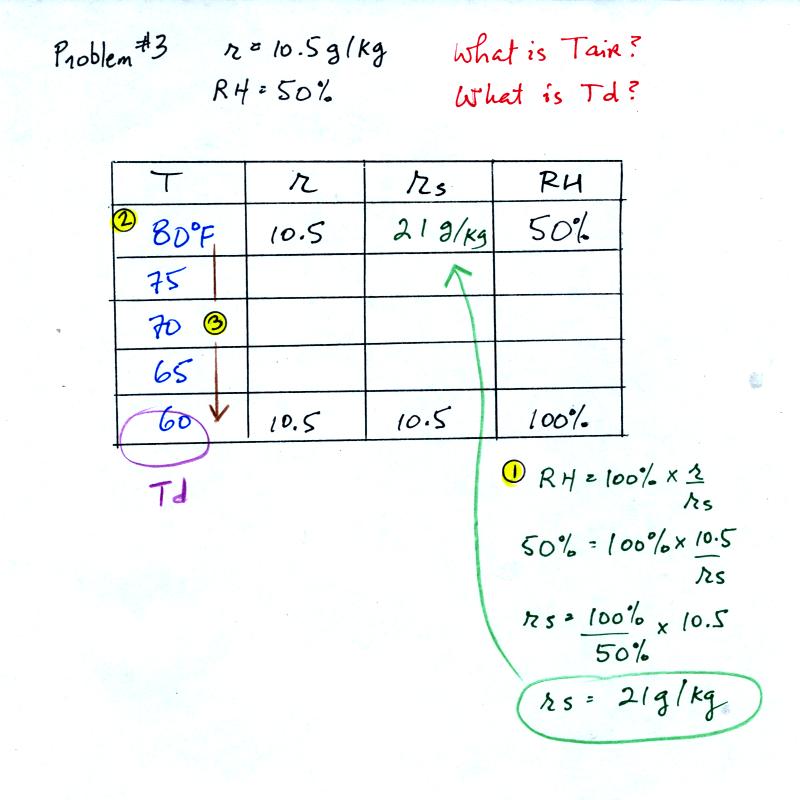
Example 3
You're given the mixing ratio (10.5) and the relative humidity
(50). What are the units (g/kg and %). Here's the play by
play solution to the question
You are given a
mixing ratio
of 10.5 g/kg and a relative humidity of 50%. You need to figure
out the air temperature and the dew point temperature.
(1) The air contains 10.5 g/kg of water vapor, this is 50%,
half, of what the air
could potentially hold. So the air's capacity, the saturation
mixing ratio must be 21 g/kg (you can either do this in your head or
use the RH equation following the steps shown).
(2) Once you know the saturation mixing
ratio you can look up the air temperature in a table (80 F air has a
saturation mixing ratio of 21)
(3) Then you
imagine cooling the air until the RH becomes 100%. This occurs at
60 F. The dew point is 60 F.
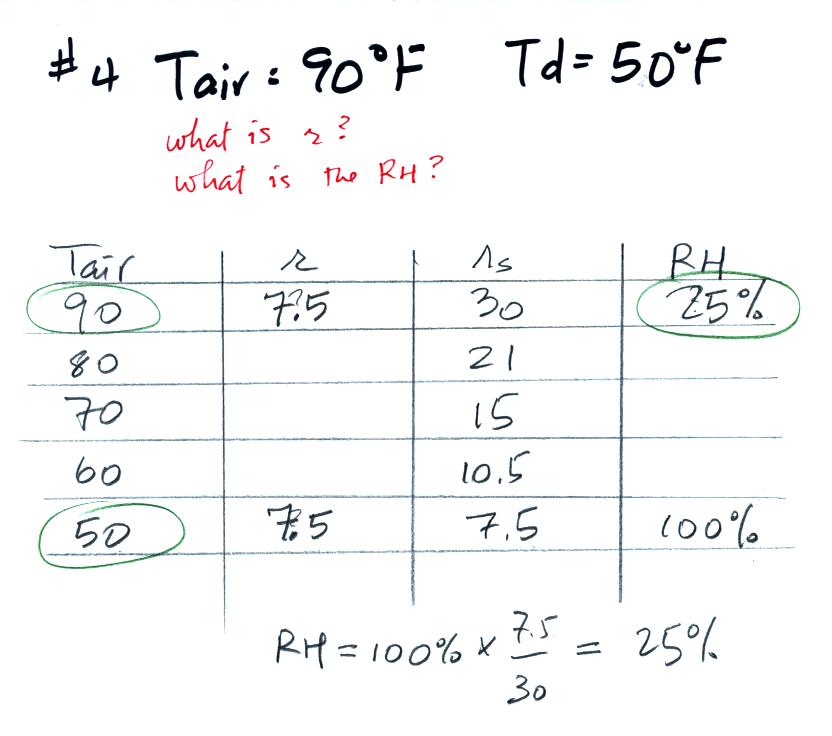
Example 4
probably the most difficult problem of the bunch.
Here's what we did in class, we
were given the air temperature and the dew point temperature. We
were supposed to figure out the mixing ratio and the relative
humidity.
We enter the two temperatures onto a chart and look up the
saturation
mixing ratio for each.
We ignore the fact that we don't know the mixing
ratio. We do know that if we cool the 90 F air to 50 F the RH
will
become
100%. We can set the mixing ratio equal to the value of the
saturation mixing ratio at 50 F, 7.5 g/kg.
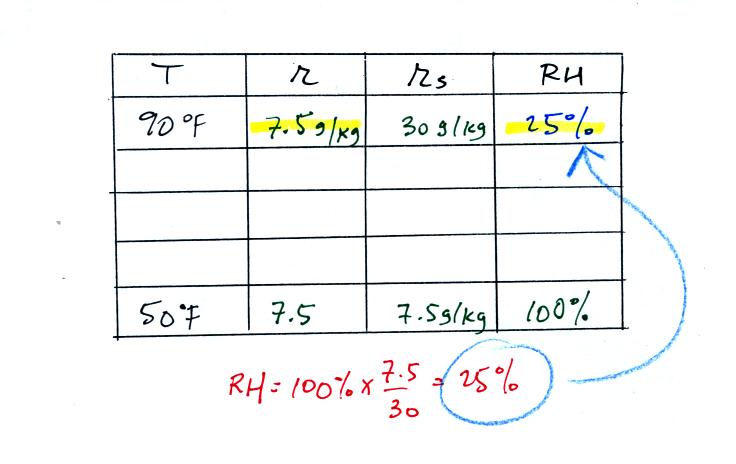
Remember back to the three earlier examples. When we
cooled air
to the the dew point, the mixing ratio didn't change. So the
mixing ratio must have been 7.5 all along. Once we know the
mixing ratio in the 90 F air it is a simple matter to calculate the
relative humidity, 25%.
Here's a list of some of the important facts and properties of the
4 humidity variables that we have been talking about.
After working through the 4 humidity problem examples you should
be
able to answer the following questions. This is the first of two
questions that formed part of an In-class Optional
Assignment. If you weren't in class, answer this question
& Question 2 below
and turn in your work at the beginning of class on Tuesday to receive
credit.
Question #1

Question #2
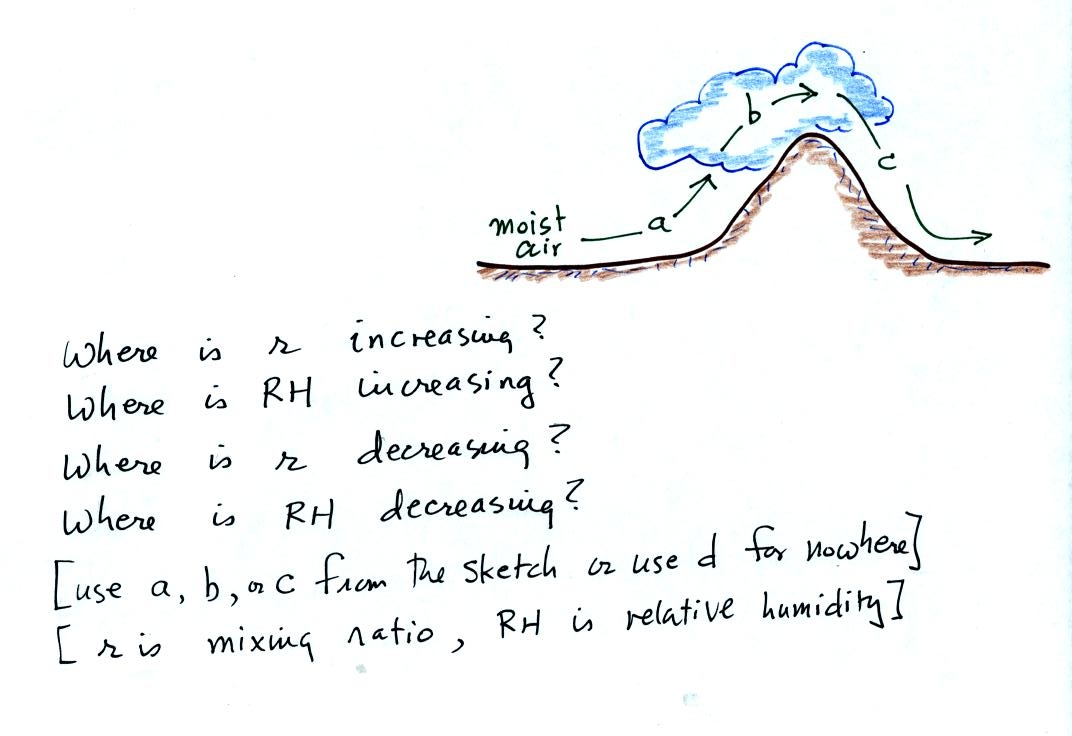
Now we can
start to use what we have learned about humidity
variables (what they tell you about the air and what causes them to
change value) to learn some new things. The figure below is on p.
87 in the photocopied ClassNotes.
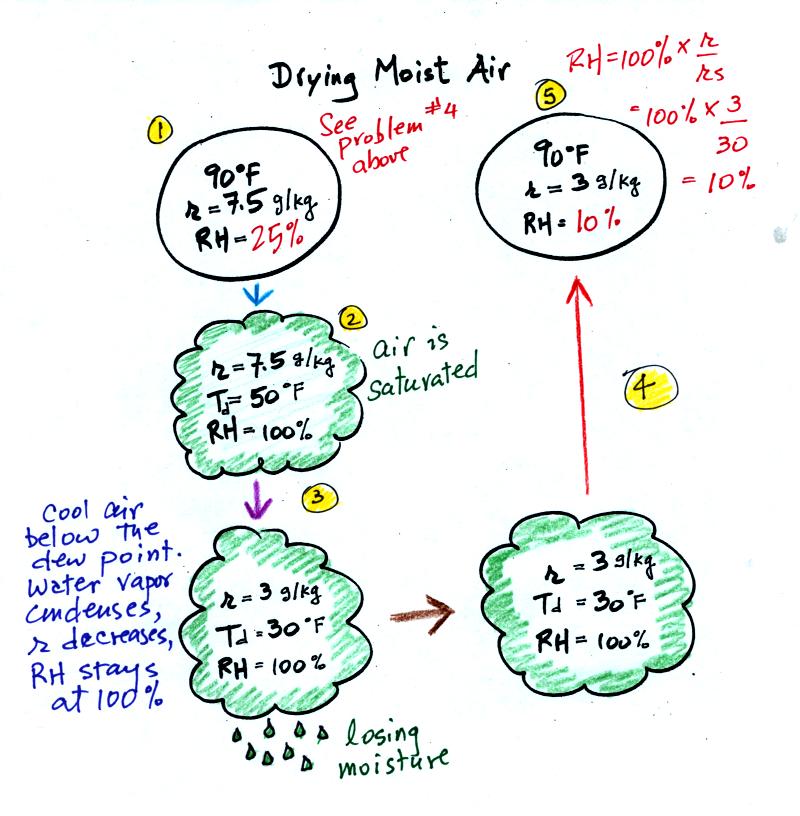
At Point 1 we start with some 90 F air with a relative
humidity of 25%, fairly dry air (these data are the same as in Problem
#4 on Monday). Point 2 shows the air being cooled to the dew
point, that is
where the relative humidity would reach 100% and a cloud would form.
Then the air is cooled below the dew
point, to
30 F. Point 3 shows the 30 F air can't hold the 7.5 g/kg of water
vapor that
was originally found in the air. The excess moisture must
condense (we will assume it falls out of the air as rain or
snow). When air reaches 30 F it contains 3 g/kg, less than half
the
moisture that it originally did (7.5 g/kg). Next, Point
4, the 30
F air is warmed back to 90 F, the starting temperature, Point 5.
The air
now
has a RH of only 10%.
Drying moist air is like wringing moisture from a wet sponge.
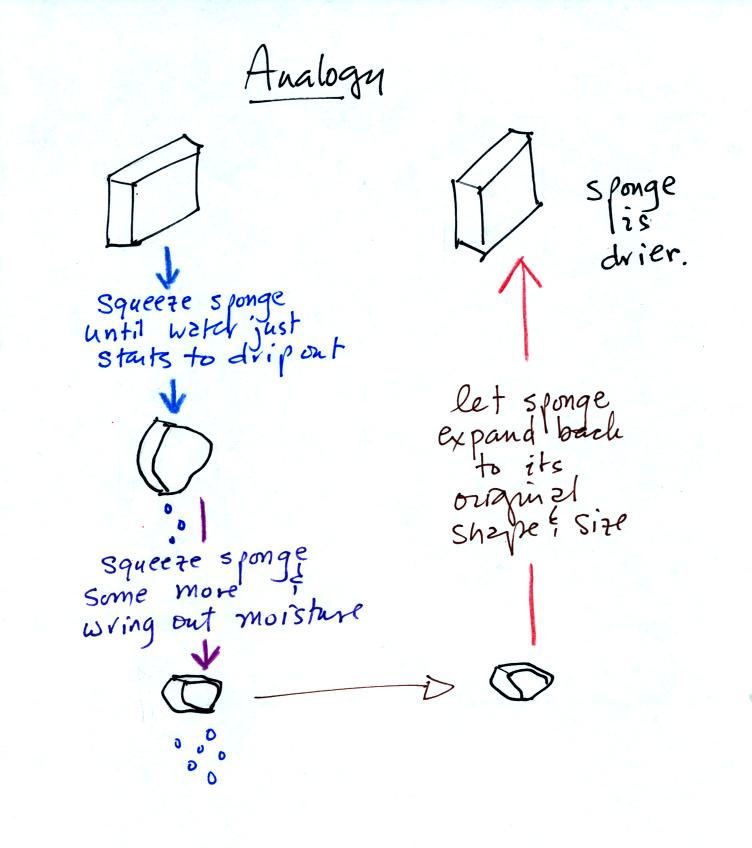
You start to
squeeze the sponge and nothing happens at first (that's like cooling
the air, the mixing ratio stays constant as long as the air doesn't
lose any water vapor). Eventually water will start to drop from
the sponge (with air this is what happens when you reach the dew point
and continue to cool the air below the dew point). Then you let
go of the sponge and let it expand back
to its orignal shape and size (the air warms back to its original
temperature). The sponge (and the air) will be drier than when
you started.
This sort of process ("squeezing" water vapor out of moist air by
cooling the air below its dew point) happens all the time. Here
are a couple of examples (p. 87 again)
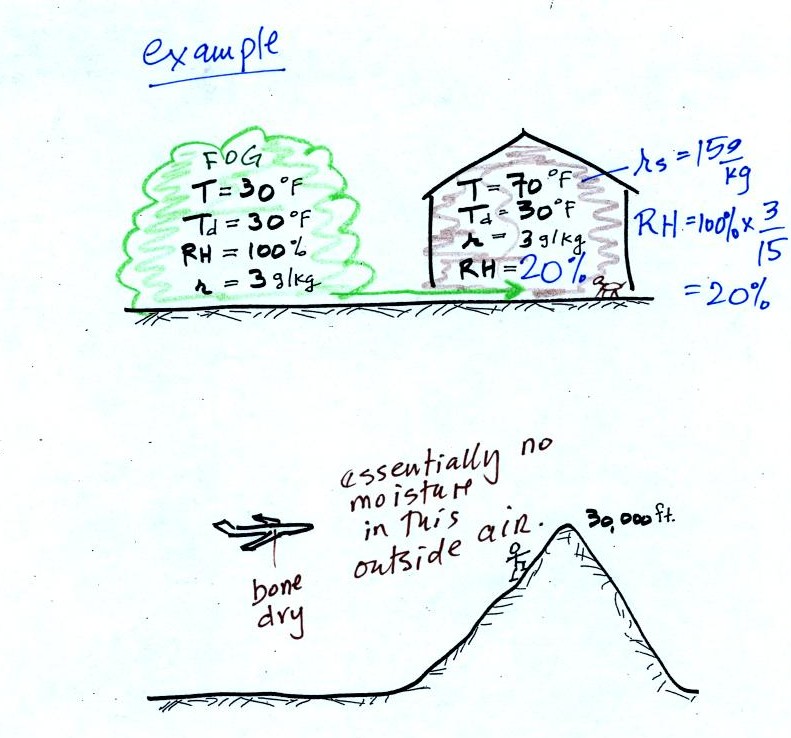
The air in an
airplane comes from outside the plane. The air outside the plane
can be very cold (-60 F perhaps) and contains very little water
vapor (even if the -60 F air is saturated it would contain essentially
no water vapor). When brought inside and warmed to a
comfortable
temperature, the RH of the air in the plane will be very close
0%.
Passengers often complain of becoming dehydrated on long airplane
flights. The plane's ventilation system probably adds moisture to
the
air so that it doesn't get that dry.
Here's a very important example, the rain shadow effect (p.
88 in the ClassNotes).
We didn't have enough time to cover this is class. I've included
it in the online notes because you might be able to use some of what
you learn here to answer Question #2 on the In-class Optional
Assignment.
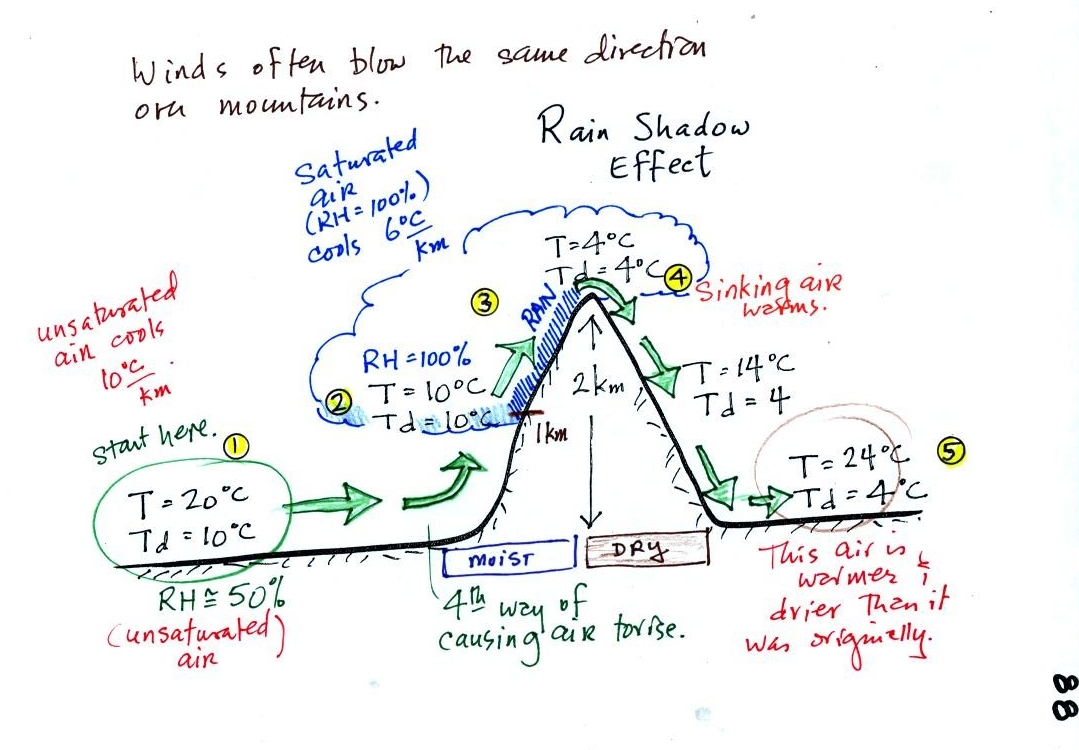
We start with some moist but unsaturated air (RH is about
50%) at Point
1.
As it is moving toward the right the air runs into a mountain and
starts to rise (see the note below). Rising air expands and cools
(see the end of today's notes for more information on this
point). Unsaturated air
cools 10 C for every kilometer of altitude gain.
This is known as the dry adiabatic lapse rate. So in rising 1 km
the air will cool to 10 C which is the dew point.
The air becomes saturated at Point 2, you would see a cloud
appear. Rising saturated air cools at a slower rate than
unsaturated air (condensation of water vapor releases latent heat
energy, this warming partly offsets the cooling caused by
expansion). We'll use a value of 6 C/km (an average
value). The air cools from 10 C to 4
C in next kilometer up to the top of the mountain. Because the
air is being cooled below its dew point at Point 3, some of the water
vapor will condense and fall to the ground as rain. Moisture is
being removed from the air.
At Point 4 the air starts back down the right side of the
mountain. Sinking air is compressed and warms. As soon as
the air starts to
sink and warm, the relative humidity drops below 100% and the cloud
disappears. The sinking unsaturated air will warm at the 10 C/km
rate.
At Point 5 the air ends up warmer (24 C vs 20 C) and drier (Td =
4 C vs Td = 10 C) than when it started out. The downwind side of
the mountain is referred to as a "rain shadow" because rain is less
likely there than on the upwind side of the mountain. Rain is
less likely because the air is sinking and because the air on the
downwind side is drier than it was on the upslope side.
Most of the year the air that arrives in Arizona comes from the Pacific
Ocean. It
usually isn't very moist by the time it reaches Arizona because it has
travelled up and over the
Sierra Nevada mountains in
California and the Sierra Madre mountains further south in
Mexico. The air loses much of its moisture on the western slopes
of those mountains.
Next in
our potpourri of topics today was measuring
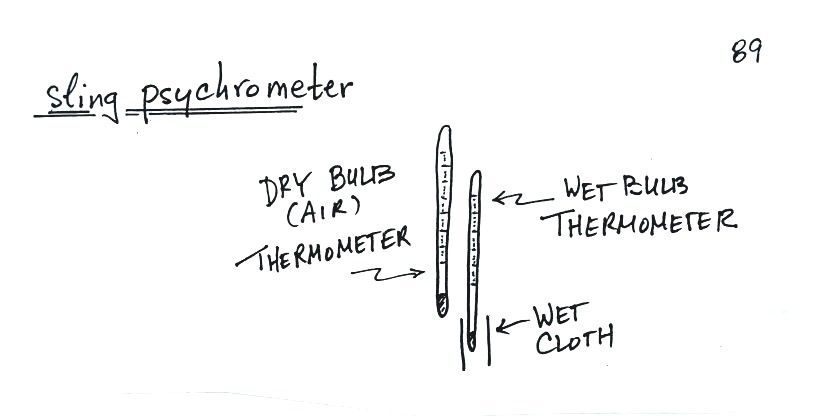
humidity. One of the ways of measuring humidity is to use a sling
(swing might be more descriptive) psychrometer.
A sling
psychrometer consists of two thermometers mounted
side by side. One is an ordinary thermometer, the other is
covered with a wet piece of cloth. To
make a humidity measurement you swing the psychrometer around for a
minute or two and then read the temperatures from the two
thermometers. The dry - wet thermometer (dry and wet bulb)
temperature difference can be
used to determine relative humidity and dew point.

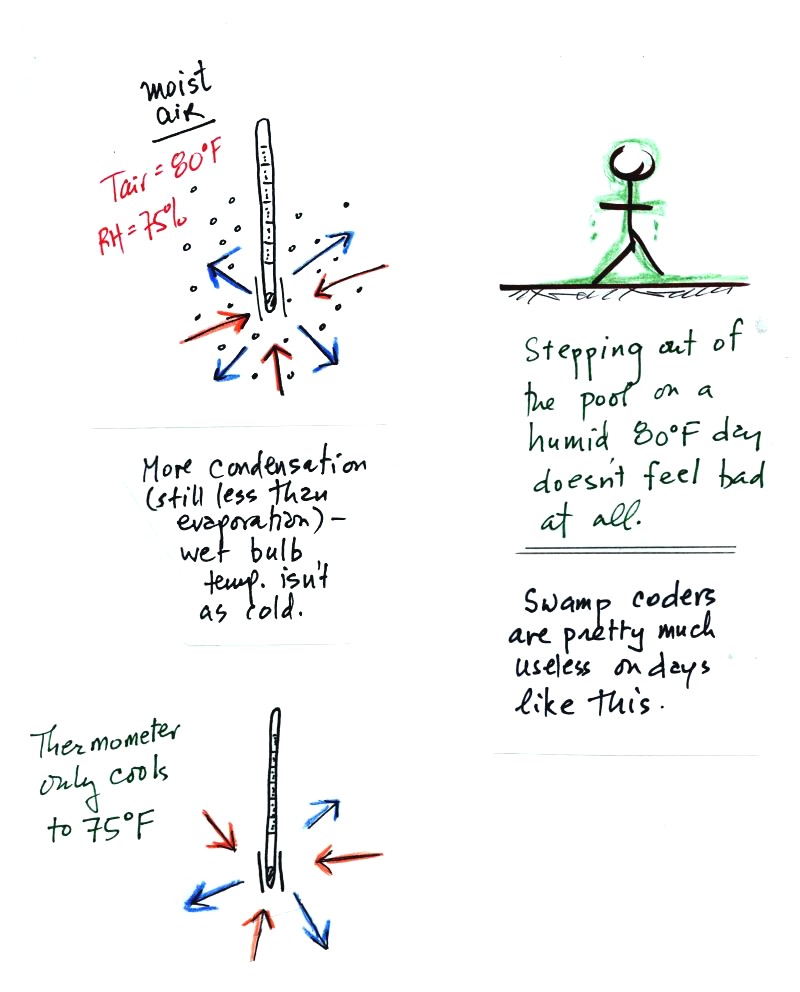
The evaporation is shown as blue arrows because this will cool the
thermometer. The same thing would happen if you were to step out
of a swimming pool on a warm dry day, you would feel cold. Swamp
coolers would work well on a day like this.
The figure at upper left also shows one arrow of condensation.
The amount or rate of condensation
depends on how much water vapor is
in the air surrounding the thermometer. In this case (low
relative humidity) there isn't much water vapor. The
condensation arrow is orange because the condensation will release
latent heat and warm the thermometer.
Because there is more evaporation (4 arrows) than condensation (1
arrow) the wet bulb thermometer will drop.
The wet thermometer will cool but it won't cool indefinitely. We
imagine that the wet bulb thermometer
has cooled to 60 F. Because the wet piece of cloth is cooler,
there is less evaporation. The wet bulb thermometer has cooled to
a temperature where the evaporation and condensation are in
balance. The thermometer won't cool any further.
You
would measure a large difference (20 F) between the dry and wet bulb
thermometers on a day like this when the air is relatively dry.
The air temperature is the same in this
example, but there is more
water vapor in the air.
You wouldn't feel as cold if you stepped out of a pool on a warm humid
day like this. Swamp coolers wouldn't provide much cooling on a
day like this.
There are four arrows of evaporation (because the water temperature is
still 80 F just as it was in the previous example) and three arrows now
of
condensation (due to the increased amount of water vapor in the air
surrounding the thermometer). The wet bulb thermometer will cool
but won't get as
cold as in the previous example.
The wet bulb thermometer might well only cool to 75 F. This might
be enough to lower the rate of evaporation (from 4 arrows to 3 arrows)
enough to bring it into
balance with the rate of condensation.
You would measure a small difference (5 F) between the dry and wet bulb
thermometers on a humid day like this.
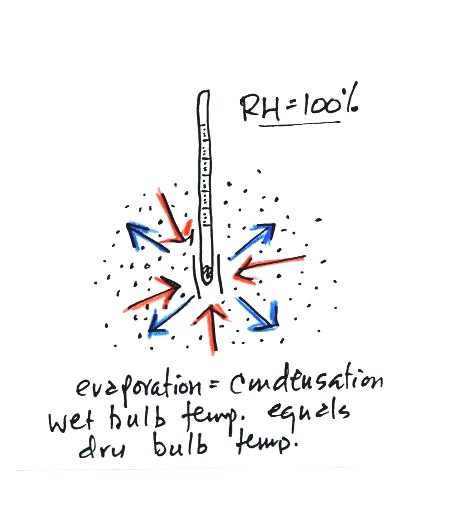
There won't be any difference in
the dry and wet bulb temperatures when
the
RH=100%. The rates at which water is evaporating and water vapor
is condensing are equal. That's one of the things that happens
when air is saturated. The dry and wet bulb thermometers would
both read 80 F.
The last thing we covered was the formation of dew, frost, and
something called frozen dew. The
figures below were redrawn after class to make them clearer.
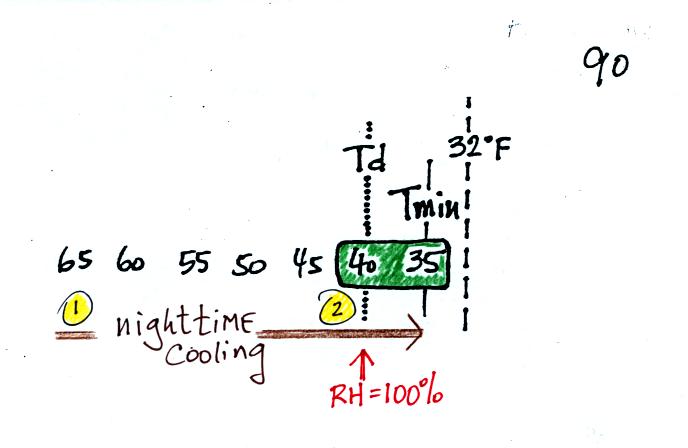
It might be a little hard to figure out what is being
illustrated
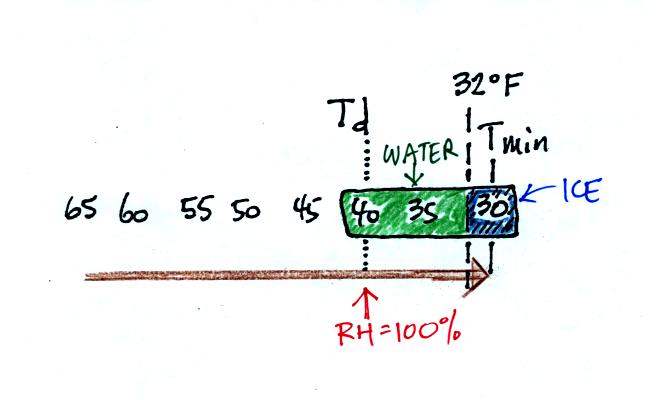
here. Point 1 is sometime in the early evening when the
temperature of the air at ground level is 65. By the next morning
the air has cooled to 35 F. When the air temperature reaches 40
F, the dew point, the relative humidity reaches 100% and water vapor
begins to condense onto the ground. You would find your newspaper
and your car covered with dew the next morning.
The next night is similar except that the nighttime
minimum
temperature drops below freezing. Dew forms and first covers
everything on the ground with water. Then the water freezes and
turns to ice. This isn't frost, rather
frozen dew. Frozen dew is often thicker and harder to scrape off
your car windshield than frost.
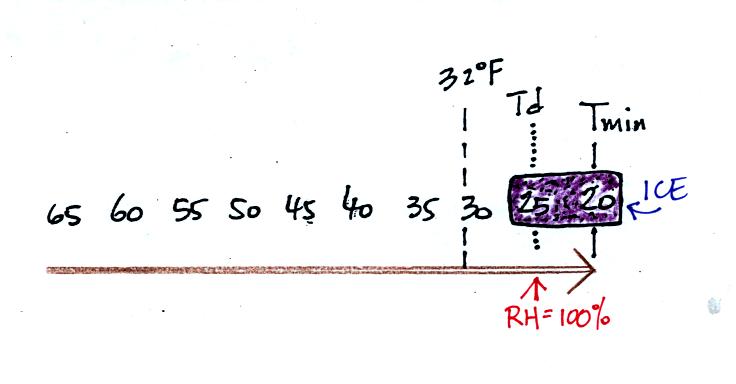
Now the dew point and the nighttime minimum temperature are both
below
freezing. When the RH reaches 100% water vapor turns directly to
ice (deposition). This is frost.
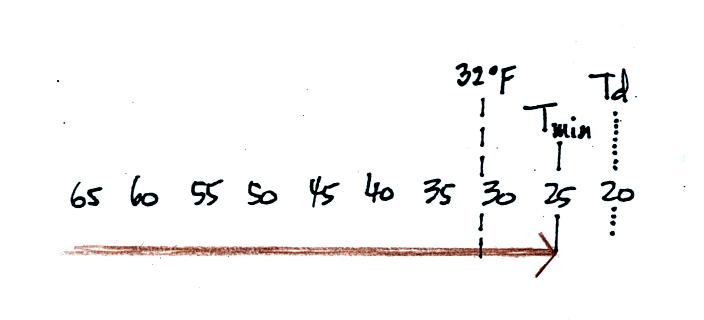
What happens on this night? Because the nighttime minimum
temperature never reaches the dew point and the RH never reaches 100%,
nothing would happen.