Thursday Oct. 7, 2010
click here to download today's notes in a more
printer friendly format
Music today featured two songs from Bob Dylan ("Like a
Rolling Stone" and "Stuck Inside of Mobile with the Memphis Blues
Again")
The Bonus 1S1P Assignment that was turned
in on Tuesday has been graded and was returned in class today (click
here to see a drawing of
the completed surface weather map analysis).
The Upper
Level
Charts
Optional
Assignment was collected today. I'll return these
papers next Tuesday. If you weren't able to
finish the assignment in time to turn it in today you can have until
next Tuesday.
The Quiz #2 Study Guide is now
online. Quiz #2 is Thursday, Oct. 14. There will be
reviews Monday, Tuesday and Wednesday afternoon next week. See
the Study
Guide for times and locations.
The Experiment #2 reports and the Expt. #1 revised reports are due next
Tuesday, Oct. 12. Try to return your materials Friday or Monday
(in my office) so that you can pick up the Supplementary
Information sheet.
An In-class
Optional
Assignment was handed out today. If you download the
assignment and turn in your answers at the beginning of class next
Tuesday
you can receive at least partial credit (maybe even full credit).
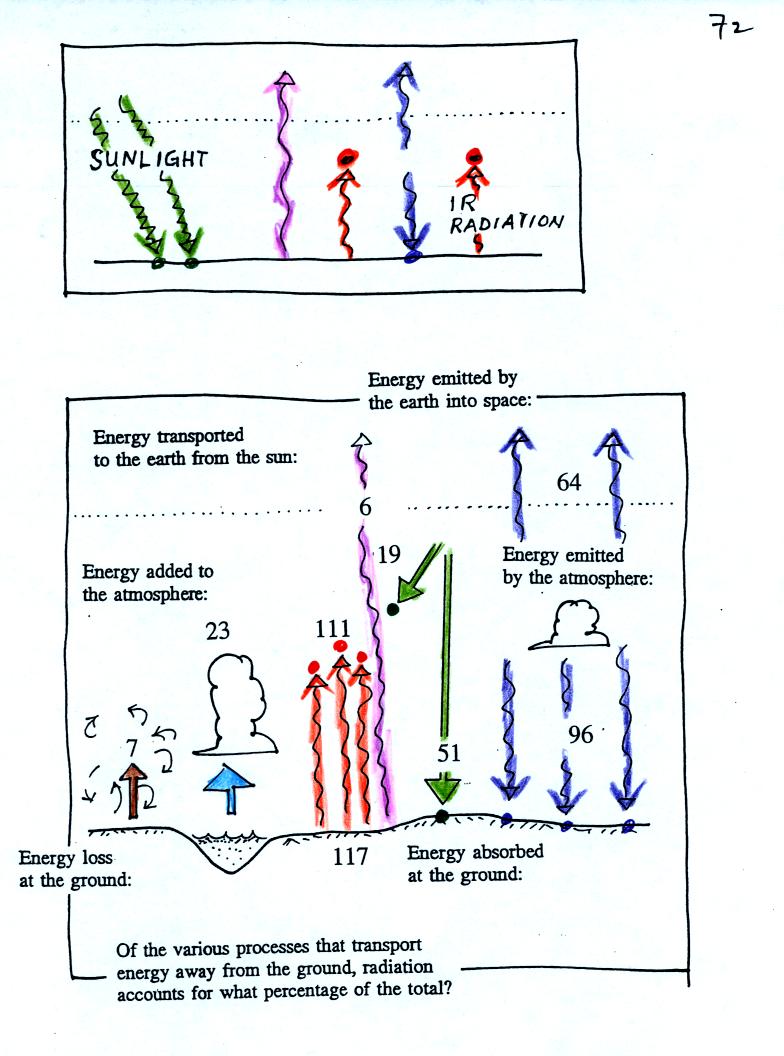
We began class with a short demonstration of Stefan Boltzmann and
Wien's Laws in action. These are the two equations that allow you
to calculate the amount and kind of electromagnetic radiation emitted
by an object.
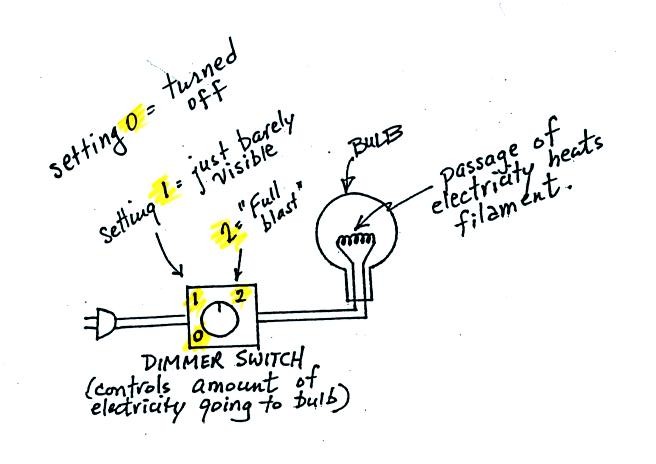
An
ordinary 200 W tungsten bulb connected to a dimmer switch was used (see
p. 66 in the photocopied
ClassNotes). We'll be seeing the EM radiation emitted by the bulb
filament.
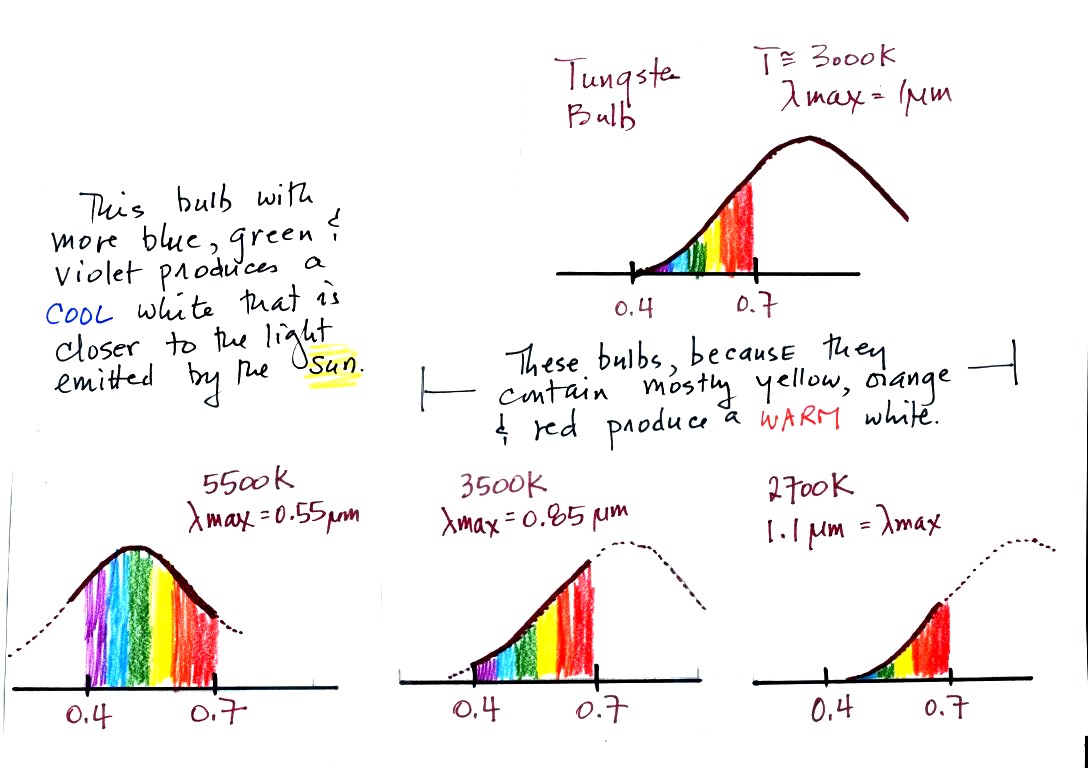
The graph at the bottom of p. 66 has been split up into 3 parts
and
redrawn for improved clarity.
We start with the bulb turned off (Setting 0).
The
filament will be at room temperature which we will assume is around 300
K (remember that is a reasonable and easy to remember value for the
average temperature of the earth's surface). The bulb will be
emitting radiation, it's shown on the top graph above. The
radiation is very weak so we
can't
feel it. The wavelength of peak emission is 10
micrometers which is long wavelength, far IR, radiation
that we aren't able to see.
Next we use the dimmer switch to just barely turn the bulb on (the
temperature of the filament is now about 900 K).
The bulb wasn't very bright at all and had an orange color. This
is curve 1, the middle figure. Note the far left end of the
emission curve has
moved left of the 0.7 micrometer mark - into the visible portion of the
spectrum. That is what you are able to see, just the small
fraction of
the radiation emitted by the bulb that is visible light (but just
long wavelength red and orange light). Most of the radiation
emitted by the bulb is to the right of the 0.7 micrometer mark and is
invisible IR radiation (it is strong enough now that you could feel it
if you put your hand next to the bulb).
Finally we turn on the bulb completely (it was a 200 Watt bulb so
it
got
pretty bright). The filament temperature
is now about 3000K. The bulb is emitting a lot more visible
light, all the colors, though not all in equal amounts. The
mixture of the colors produces a "warm
white" light. It is warm because it is a mixture that contains a
lot more red, orange, and yellow than blue, green, and violet
light. It is interesting that most of the radiation emitted by
the bulb is still in the IR portion of the spectrum (lambda max is 1
micrometer). This is
invisible light. A tungsten bulb like this is not especially
efficient, at least not as a source of visible light.
Energy
efficient
compact
fluorescent
lamps
(CFLs)
are
being
touted as an ecological alternative to tungsten bulbs because
they use substantially less electricity, don't emit a lot of
wasted infrared light, and also last
longer. CFLs come with
different color temperature ratings.
The bulb with the hottest
temperature rating (5500 K ) in the figure
above is meant to mimic or simulate sunlight. The temperature of
the sun is 6000 K and lambda max is 0.5 micrometers. The spectrum
of the 5500 K bulb is similar.
The tungsten bulb (3000 K) and the CFLs with temperature ratings
of
3500 K and 2700 K produce a warmer white.
Three CFLs with the temperature ratings above were set up in class
so
that you could see the difference between warm and cool white
light. Personally I find the 2700 K bulb "too warm," it makes a
room
seem gloomy and depressing. The 5500 K bulb is "too cool" and
creates
a stark sterile atmosphere like you might see in a
hospital. I prefer the 3500 K bulb in the
middle.
This figure below is from an article
on compact fluorescent lamps in Wikipedia for those of you that weren't
in class and didn't see the bulb display.. You can
see a clear difference between the cool white bulb on the left
in the figure below and the warm white light produced by a tungsten
bulb (2nd from the left) and 2 CFCs with low temperature ratings (the 2
bulbs at right).
There is one downside to these energy efficient CFLs. The bulbs
shouldn't just be discarded in your ordinary household trash because
they contain mercury. They should be disposed of properly (at a
hazardous materials collection site or perhaps at the store where they
were purchased).
We now
have most of the tools we will need to begin to study energy balance on
the earth. It will be a balance between incoming sunlight
energy and outgoing energy emitted by the earth. We will look at
the simplest case first, the earth without an atmosphere (or at least
an atmosphere without greenhouse gases) found on p. 68 in the
photocopied Classnotes.
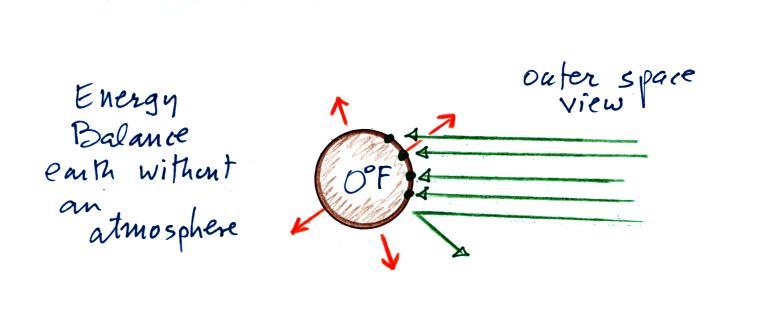
You might first wonder how, with
the sun emitting so much
more
energy than the earth, it is possible for the earth (with a temperature
of around 300 K) to be in energy
balance with the sun (6000 K). At the top right of the figure you
can see that the earth is located about 90
million miles
from the sun and therefore only absorbs a very small fraction of the
total energy emitted by the sun.
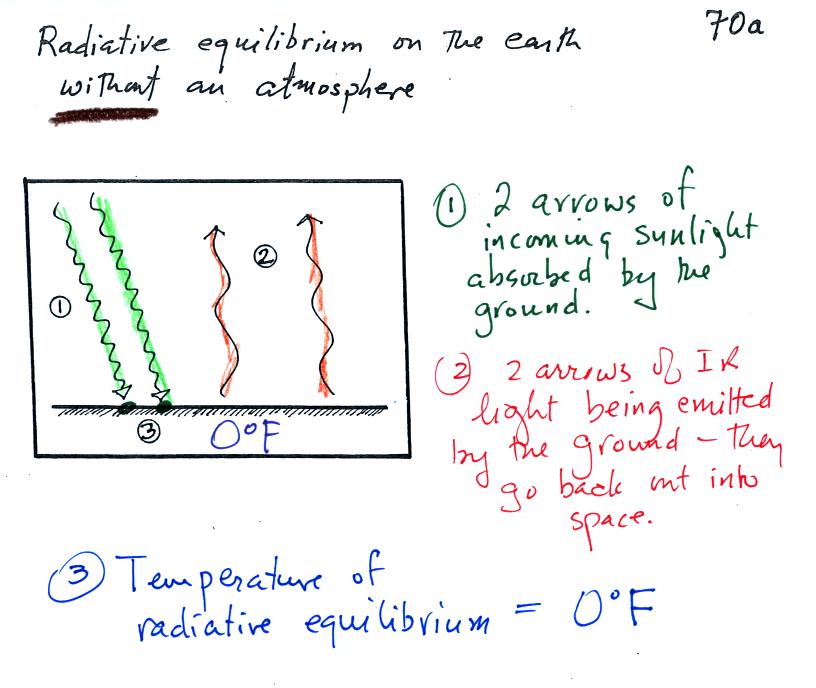
To understand how energy balance occurs we start, in Step #1, by
imagining that the earth starts out very cold (0K) and is
not emitting
any EM radiation at all. It is absorbing sunlight (4 of the 6
arrows of incoming sunlight in the first picture are absorbed, 2 of the
6 are being reflected) however so it
will
begin to warm This is like opening a bank account, the balance
will be zero. But then you start making deposits and the balance
starts to grow.
Once the earth starts to warm it will also begin to emit EM
radiation, though not as much as it is getting from the sun (the
slightly warmer earth in the middle picture is now colored blue).
The four arrows of incoming sunlight that are absorbed are shown in the
middle figure. The two arrows of reflected sunlight have been
left off because they don't really play a role in energy balance.
The earth is emitting 3 arrows of IR light (in red). Once you
find money in your bank account you start to spend it.
Because the earth is still gaining more energy than it is losing the
earth will warm some more. Reflected sunlight is like a check
that bounces. It really doesn't affect your bank account balance.
Eventually it will warm enough that the earth (now shaded green)
will
emit the same amount
of energy (though not the same wavelength energy) as it absorbs from
the sun. This is radiative equilibrium, energy balance (4 arrows
of absorbed energy are balanced by 4 arrows of emitted energy).
The
temperature at
which this occurs is about 0 F.
That is called the temperature of radiative equilibrium.
Before we
start to look at radiant energy balance on the earth with an atmosphere
we
need to learn about filters. The atmosphere will filter sunlight
as it
passes through the atmosphere toward the ground. The atmosphere
will
also filter IR radiation emitted by the earth as it trys to travel into
space.
We will first look at the effects simple blue, green, and red glass
filters have on visible light. This is just to become familiar
with filter absorption graphs.
If you try to
shine white light (a
mixture of all the colors) through a
blue filter, only the blue light passes through. The filter
absorption curve shows 100% absorption at all but a narrow range of
wavelengths that correspond to blue light. Similarly the green
and red filters only let through green and red light.
The following figure is a simplified, easier to
remember,
representation of the
filtering effect of
the atmosphere on UV, VIS, and IR light (found on p. 69 in the
photocopied notes). The figure was redrawn after class.

You can use your own eyes to tell
you what the filtering
effect of the
atmosphere is on visible light. Air is clear, it is
transparent. The atmosphere transmits visible light.
In our simplified representation oxygen and ozone make the
atmosphere pretty nearly completely opaque to UV light . We
assume that the
atmosphere absorbs all incoming UV light, none of it makes it to the
ground. This is of course not entirely realistic.
Greenhouse gases make the
atmosphere a
selective absorber of IR light - the air absorbs certain IR wavelengths
and
transmits others. It is the atmosphere's ability to absorb (and
also emit) certain wavelengths of infrared light that produces the
greenhouse effect and warms the surface of the earth.
Note "The atmospheric window"
centered at 10 micrometers and something I didn't
mention in class. Light emitted by the earth at this
wavelength (and remember 10 um is the wavelength of peak emission for
the earth) will pass through the atmosphere. Another transparent
region, another window, is found in the visible part of the spectrum.
You'll find a more realistic picture of the atmospheric absorption
curve on p. 70 in the photocopied Classnotes, but the simplified
version above will work fine for us.
Here's the
outer space view of radiative equilibrium on the earth without an
atmosphere. The important thing to note is that the earth is
absorbing and emitting the same amount of energy (4 arrows absorbed
balanced by 4 arrows emitted).
We will be moving from an outer
space vantage point of
radiative equilibrium (figure above) to the earth's
surface (figure below).
Don't let the fact that there are
4 arrows are
being absorbed and
emitted in the top figure and
2 arrows absorbed and emitted in the bottom figure
bother you. The important thing is that there are equal
amounts being absorbed and emitted in both cases.
The next
step is to add the atmosphere.
We will study a simplified
version
of radiative equilibrium just so you
can identify and understand the various parts of the picture.
Keep an eye out for the greenhouse effect. Here's something close
to what
we ended up with in class
It would be hard to sort through all of this if you weren't in
class
(and maybe even if you were) to see how it developed. So below we
will go through it again step by step (which you are free to skip over
if you wish. This is a little
more detailed version than was done in class. Caution:
some of the colors below are different
from used in class.
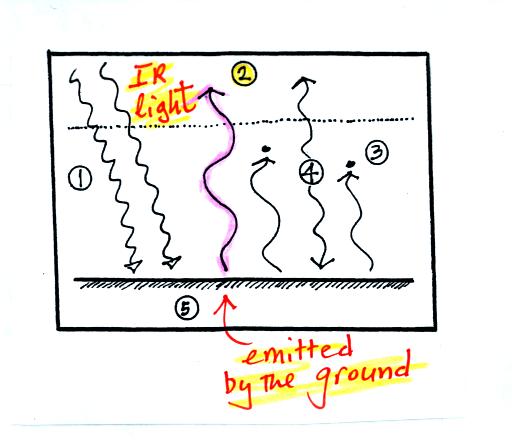
1. The
figure shows two
rays of incoming sunlight that
pass through the atmosphere, reach the ground, and are absorbed.
100% of the incoming sunlight is transmitted by the atmosphere.
This wouldn't be too bad of an assumption if sunlight were just visible
light. But it is not it is about half IR light and some of that
is going to be absorbed.
The ground is emitting
3 rays of IR radiation.
2. One
of
these
(pink
arrow
above)
is emitted by
the ground at a wavelength
that is
NOT absorbed by greenhouse gases in the atmosphere (probably around 10
micrometers). This
radiation passes through the atmosphere and goes out into space.
3. The other 2
units of IR radiation emitted by
the
ground are
absorbed by
greenhouse gases is the atmosphere.
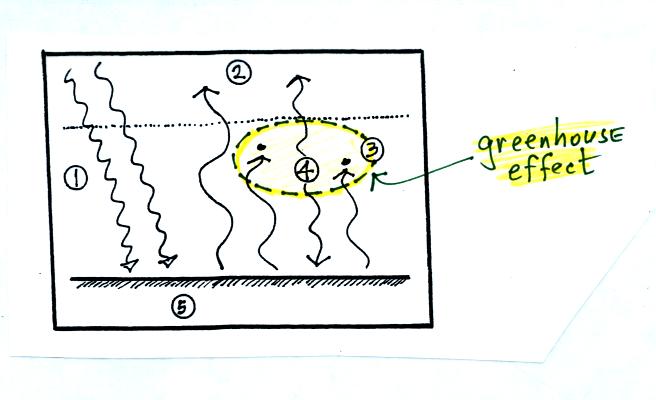
4. The
atmosphere is absorbing
2 units of radiation.
In order to be in radiative equilibrium,the atmosphere must also emit 2
units of radiation. 1
unit of IR radiation is sent upward into space, 1 unit is sent downward
to the ground where it is absorbed. This is probably the part of
the picture that most students have visualizing.
Before we go any further we will
check
to be sure that
every part
of this picture is in energy balance.
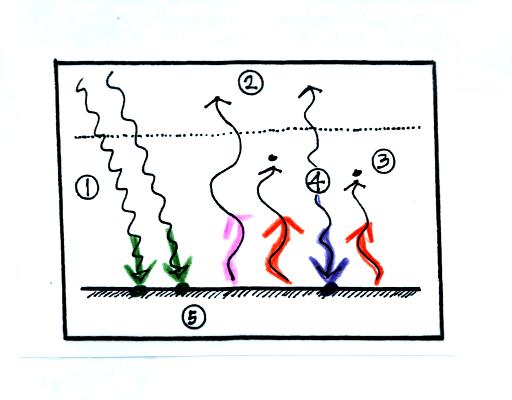
The ground is absorbing 3 units of energy (2 green
arrows of sunlight and one bluish arrow coming from the atmosphere) and
emitting
3
units of energy (one pink and two red arrows)
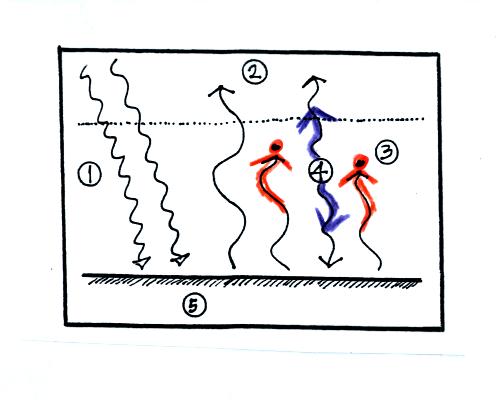
The atmosphere is absorbing 2 units of energy (the 2
red arrows coming from the ground) and
emitting 2
units of
energy (the 2 blue arrows)

And we should check to be sure equal amounts of energy
are arriving at and leaving the earth. 2 units of energy arrive
at the top of the atmosphere (green) from outer
space, 2 units
of
energy (pink and orange) leave the earth and head back out into space.
The greenhouse effect
is found in the absorption and
emission
of IR radiation by the atmosphere. Here's how you might put it
into words (close to but perhaps not exactly the same wording as in
class):
Doesn't it make sense that if the ground is getting back some of
the energy it would otherwise lose, the ground will end up being
warmer. That's what the greenhouse effect does, it warms the
earth. The global annual average surface temperature is about 60
F on the earth with a greenhouse effect. It would be about 0 F
without the greenhouse effect.
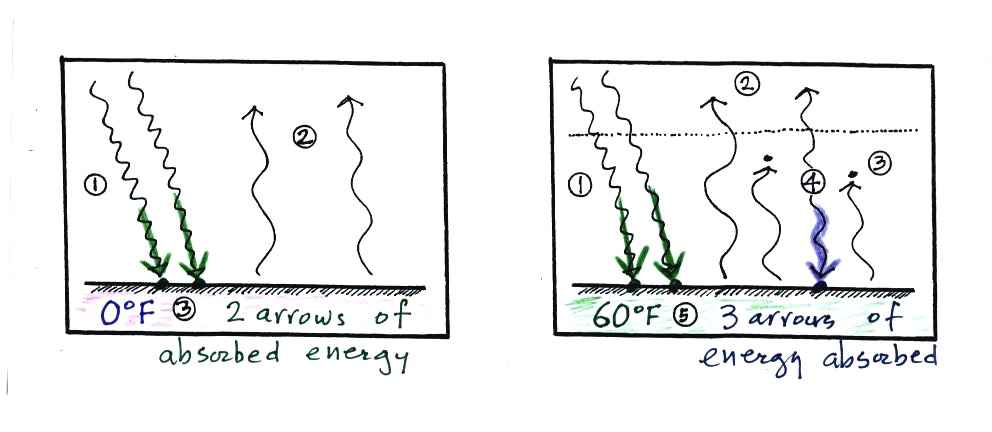
Here are a couple other ways of understanding why this is true.
Energy balance without
an atmosphere (left) and with an atmosphere that contains greenhouse
gases (right) the
greenhouse
effect. At left the ground is emitting 2 units of energy, at
right the ground is emitting 3 units. Remember that the amount of
energy emitted by something depends on temperature. The ground
in the right picture must be warmer to be able to emit 3 arrows of
energy rather than 2
arrows.
Here's another
explanation (not mentioned in
class).
At left the ground
is getting 2 units of energy (from the sun). At right it is
getting three, two from the sun and one from the atmosphere.
Doesn't it seem
reasonable
that ground that absorbs 3 units of energy will be warmer than ground
that is only absorbing 2?
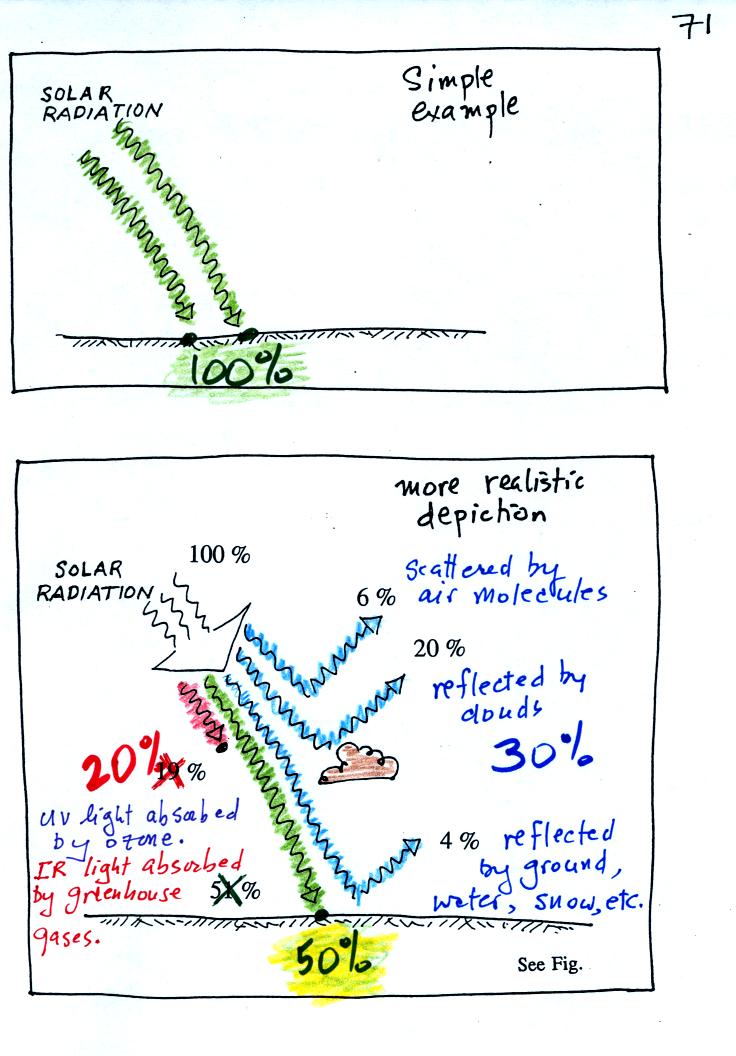
In
our simplified explanation of the greenhouse effect we assumed that
100% of the sunlight arriving at the earth passed through the
atmosphere and got absorbed at the ground. Next we will look at how
realistic that assumption is.
The bottom figure
above shows that on average (over the year and over the globe) only
about 50% of the incoming sunlight
makes it through the atmosphere and gets absorbed at the ground.
About 20% of the incoming sunlight is absorbed by gases in the
atmosphere. Sunlight is a
mixture of UV, VIS, and IR light.
Ozone and oxygen will absorb a lot of the UV (though there isn't much
UV in sunlight) and greenhouse gases will absorb some of the IR
radiation in sunlight (Roughly half of sunlight is IR light).
The remaining 30% of the incoming sunlight is reflected or
scattered back into
space
(by the ground, clouds, even air molecules).
Student performing Experiment #3 will be measuring the amount of
sunlight energy arriving at the ground. About 2 calories pass
through a square centimeter per minute at the top of the
atmosphere. Since about half of this arrives at the ground on
average, students should expect to get an answer that is about 1
calorie/cm2 min.
Next we
will look at our simplified version of radiative equilibrium and a more
realistic picture of the earth's energy budget.
In the top figure (the simplified
representation of energy balance)you should recognize the incoming
sunlight
(green),
IR emitted by the ground that passes through the atmosphere (pink), IR
radiation emitted by the ground that is absorbed by greenhouse gases in
the atmosphere (orange) and IR radiation emitted by the atmosphere
(dark blue). Using the colors you can find each of these parts of
the energy budget in the bottom figure. Notice that conduction,
convection, and latent heat energy transport are needed to bring the
overall energy budget into balance. The amount of energy transported by
conduction, convection, and latent heat is small compared to what is
transported in the form of EM radiation.
The lower part of the figure is
pretty complicated. It
would be
difficult to start with this figure and find the greenhouse effect in
it. That's why we used a simplied version. Once you
understand the upper figure, you should be
able to find and understand the corresponding parts in the lower figure.
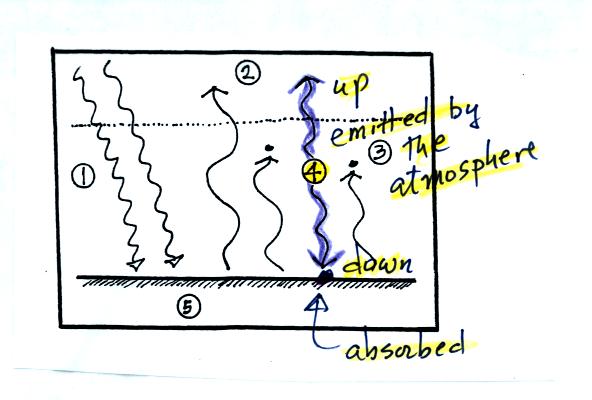
Two or three things to note in the bottom (not mentioned in
class on Thursday)
(i) First
the ground receives more energy from the atmosphere (96 units) than it
gets from the sun (51 units). Part of the reason for this is
that the sun just shines for part of the day. We receive energy
from the atmosphere 24 hours per day.
(ii) The ground emits more energy (117
units) than it gets from the sun (51 units). It is able to
achieve energy balance because it gets lots of energy from the
atmosphere.
(iii) The atmosphere emits 64 units upward and 96 units
downward. This might be explained by the lower atmosphere being
warmer than higher up in the atmosphere. Part of the explanation
is also that there is more air in the bottom of the atmosphere than
near the top of
the atmosphere.