Thursday Sep. 2, 2010
click here to download today's notes in a more
printer friendly format.
Several songs ("La Vida Tombola", "Me Gustas Tu", "Besoin de la
Lune", and "Y Ahora Que?" are the ones I
remember) from Manu Chao
before class today.
The Practice Quiz is one week from today. A Study Guide is now available online.
Reviews have also been scheduled for Tuesday and Wednesday afternoon
next week.
Hurricane Earl, now a Category 4 hurricane (on a scale that goes to 5),
is forecast to brush the East Coast this week and weekend. You
can find the latest news and predicted storm path at the National Hurricane Center website.
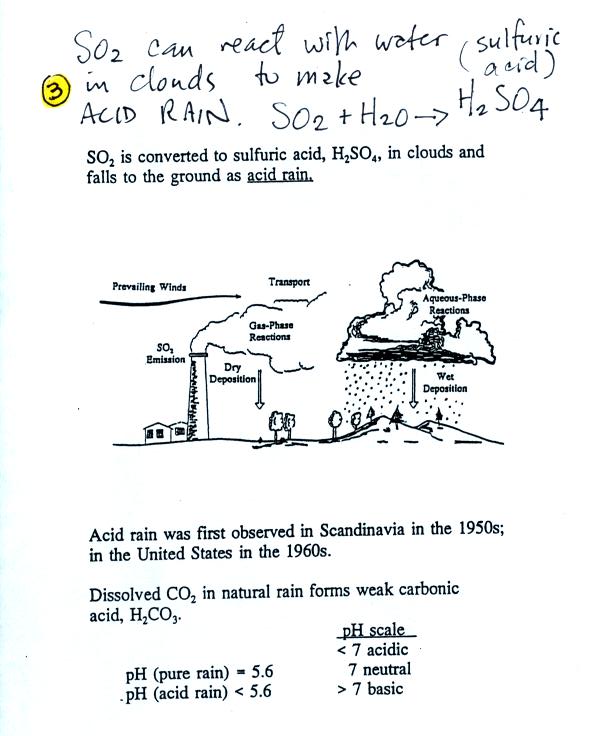
Sulfur
dioxide is one of the pollutants that can react with water in
clouds to form acid rain (some of the oxides of nitrogen react
with
water to form nitric acid). The formation and effects of acid
rain
are discussed on p. 12 in the photocopied Class Notes.
Acid rain is often a problem in regions that are
100s even 1000s of miles from the source of the sulfur dioxide.
Acid rain in Canada could come from sources
in the US, acid rain was first observed in Scandinavia and resulted
from SO2 emissions in industrialized areas in other parts of Europe.
Note at the bottom of the figure above that clean unpolluted rain has
a pH less than 7
and is
slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. The acid rain demonstration done in class today
will reinforce this point.
Some of the problems associated with acid rain are listed
above. Click on
this acid
rain demonstration
link for a detailed discussion of the demonstration done in class.
The last
pollutant that we will cover is Particulate Matter (PM) - small solid
particles or drops of liquid that remain suspended in
the air (sometimes referred to as aerosols). The designations
PM10
and PM25 refer to particles with
diameters less than 10 micrometers and 2.5 micrometers,
respectively. A micrometer is one millionth of a meter. The
drawing below might give you some idea of what a 1 micrometer particle
would look like (actually it would probably be too small to be seen
without magnification).
Particulate matter can be produced
naturally (wind blown dust,
clouds above volcanic eruptions, smoke from lightning-caused forest and
brush fires). Human activities also produce particulates.
Particles with dimensions of 10 micrometers and less can be
inhaled
into the lungs (larger particles get caught in the nasal
passages). Inhaled particulates are a health threat. The
particles can cause cancer, damage lung tissue, and aggravate existing
repiratory diseases. The smallest particles can pass through the
lungs and get into the blood stream (just as oxygen does) and damage
other organs in the body.
The figure below identifies some of the parts of the human lung
mentioned
in the figure above. Here's a link to the Bodies Exhibition
in downtown Tucson that I mentioned in class.
Crossectional view of the
human lungs
from: http://en.wikipedia.org/wiki/Lung
|
1 - trachea
2 - mainstem bronchus
3 - lobar bronchus
4 - segmental bronchi
5 - bronchiole
6 - alveolar duct
7 - alveolus
from http://en.wikipedia.org/wiki/Image:Illu_quiz_lung05.jpg
|
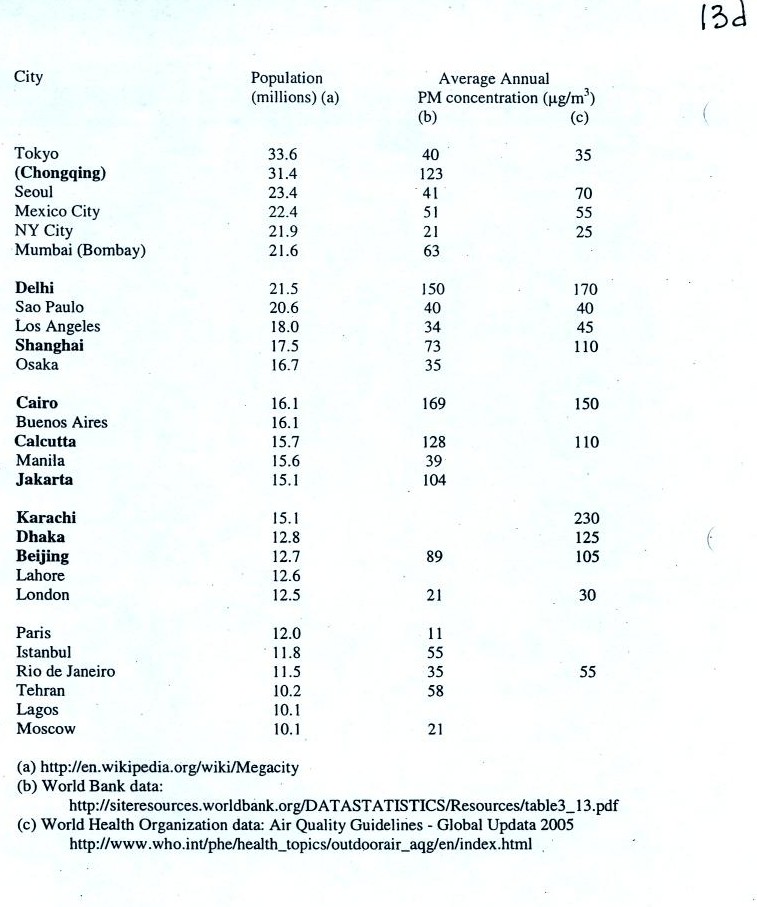
Note the
PM10 annual National
Ambient Air Quality Standard (NAAQS) value of 50 micrograms/meter3 at
the bottom of p. 13c in the photocopied ClassNotes (above).
The following list (p. 13d in the ClassNotes) shows that there are
several cities around the world
where PM concentrations are 2 or 3 times higher than the NAAQS
value.
There was some concern during the
summer 2008 Olympic Games
that the polluted air in
Beijing would keep athletes from performing at their peak.
Chinese authorities restricted transportation and
industrial activities both before and during the games in an attempt to
reduce pollutant concentrations. Rainy weather during the games
may have had the greatest effect, however.
Clouds and precipitation are the
best way of cleaning pollutants from the air. We'll see later in
the semester that cloud droplets form on small particles in the air
called condensation nuclei. The cloud droplets then form
raindrops and fall to the ground carrying the particles with
them. Some of the gaseous pollutants dissolve in water and can
also be removed by clouds and precipitation.
Particulate matter can also affect visibility. We'll look at how
and why that happens at the end of today's lecture notes. At this
point we took a brief detour and covered a little new material on
stratospheric ozone. Stratospheric ozone (the ozone layer)
absorbs dangerous high-energy ultraviolet light. Tropospheric
ozone is
a pollutant and a key ingredient in photochemical smog.
This information is covered on pps 17 & 18 in the photocopied
ClassNotes.
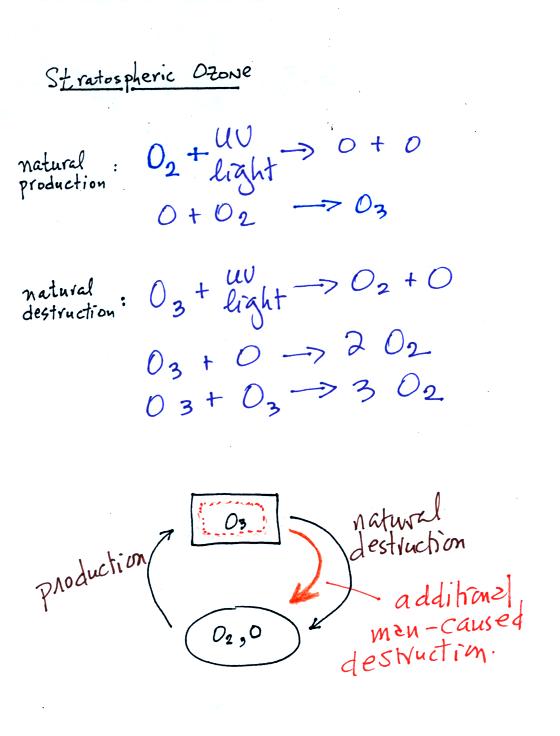
The
top
two
equations
show
how ozone is produced in the stratosphere.
Ultraviolet (UV) light splits an O2 molecule into two O atoms.
Each of the O atoms can react with O2 to make O3 (ozone).
Ozone is destroyed when it absorbs UV light and is split into O and O2
(the two pieces move away from each other and don't recombine and
remake ozone). O3 is also destroyed when it reacts with an oxygen
atom (thereby removing one of the "raw ingredients" used to make
ozone). Two molecules of ozone can also react to make 3 molecules
of O2.
The bottom part of the figure attempts to show that the ozone
concentration in the
stratosphere will shift up and down until the natural rates of
production and
destruction
balance each other (analogous to your bank account not changing when
the amount of money deposition and withdrawn are equal). If an
additional man-caused destruction process is added (orange) that will
lower the ozone layer concentration (if someone else starts spending
some of your money, you balance will decrease).
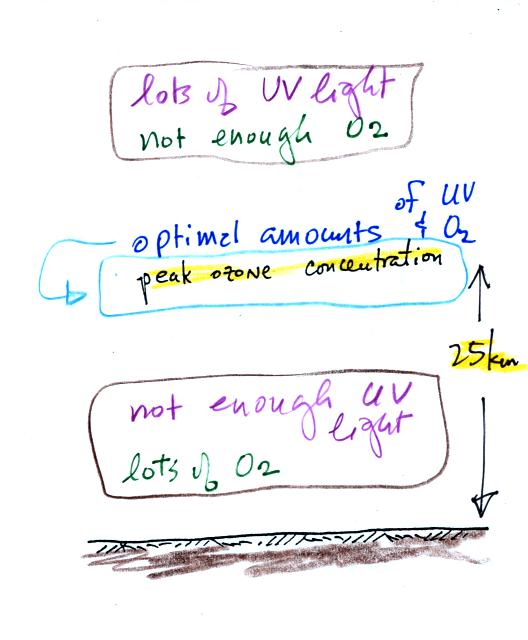
Knowing that you need O2 and UV light to make ozone, you can
begin
to understand why the ozone layer is found in the middle of the
atmosphere.
There is plenty of UV light high in the atmosphere but not much
oxygen (air gets thinner at higher and higher altitude). Near the
ground there is plenty of oxygen but not as much UV light (it is
absorbed by gases above the ground). You find the optimal amounts
of UV light and oxygen somewhere in between, near 25 km altitude.
This next figure lists some of the problems associated with
exposure to UV light. Thinning of the ozone layer will result in
increased amounts of UV light reaching the ground.
Skin cancer and cataracts are probably the best known hazards
associated with UV light.
Human activities add substances to the atmosphere that can
potentially
reduce ozone concentration in the ozone layer (which would result in
increased exposure to UV light at the ground).
The first set of reactions above involve nitric oxide, NO. First,
NO reacts with O3 to form NO2 and O2
(ordinary molecular oxygen).
Then notice the NO2 reacts with an oxygen atom (which might
otherwise
react with O2 to make O3) to form NO again and O2.
The
NO
is
available
again
to
react
with and destroy another ozone molecule.
At one time many countries were considering building fleets of
supersonic aircraft that would fly into the stratosphere. The
plans were scrapped partly due to concern that the NO emissions from
these aircraft would damage the ozone layer.
The main threat now comes from chlorofluorocarbons (CFCs). CFCs
were
at
one
time
thought
to be an ideal industrial
chemical and had a variety of uses.
CFCs are unreactive, non toxic, and stable. Once they get into
the atmosphere they remain there a long time, as much as 100 years.
The
reactions
involving
CFCs
are
shown
on the next figure.
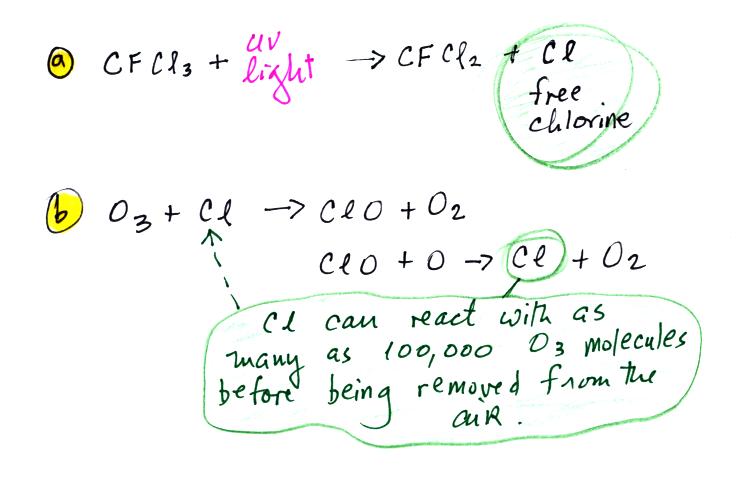
CFCs released at ground level remain in the atmosphere long
enough that they can eventually make
their way up into the stratophere. UV light can then break
chlorine
atoms off the CFC molecule [a]. The resulting
"free chlorine" can react with and destroy ozone. This is shown
in [b] above. Note how the chlorine atoms reappears at the end of
the two step reaction. A single chlorine atom can destroy 100,000
ozone molecules.
OK back to particulate matter and the effect it has on
visibility. Particulates
can reduce visibility and make the sky appear hazy. To
understand this we need to look at how air molecules and particles
scatter sunlight.

Rays of sunlight are passing
through clean air above. To see the sunlight you would need to
look back toward the sun along one of the rays (direction a) in the
figure (this would be like standing next to the spot on the wall during
the laser demonstration and looking back at the laser). You'd see
the sun in that case (you shouldn't of course look directly at the
sun). If you look away from the sun and at the sky (direction b)
you see blue light. This is sunlight that is being scattered by
air molecules.
Sunlight is white light, a mixture of all the
colors. Because air molecules are small (relative to the
wavelength of visible light) they scatter shorter wavelengths more
readily than longer wavelengths. When you look away from the sun
and toward the sky you see this scattered light, it has a deep blue
color. This is basically why the sky is blue. If the earth
didn't have an atmosphere (or if air molecules didn't scatter light)
the sky would be black (you wouldn't see any scattered light).
We've added some particles to the
air in the picture below. Particles also scatter light
(remember the chalk dust used in the laser demonstration).
Particles are much bigger than air molecules. When the particle
size
is about equal to or somewhat greater
than the wavelength of visible light the particles scatter all the
colors equally. The light scattered by particles is white.
Clouds are white because the cloud droplets are relatively large and
scatter all the colors in sunlight equally.
When you look at the
sky now you see blue light from sunlight scattered by air molecules
mixed together with some white light that is sunlight being scattered
by particles. The color of the sky
changes from deep blue to bluish white. The higher the
particle concentration, the whiter the sky becomes.
Pay attention to the color of the sky before and after a rainstorm if
predicted to move through Tucson.
Before the storm, the air will be full of particulate pollution and
will appear whitish blue. After the storm, after the rain has
removed a lot of the pollutants, the sky often has a much deeper blue
color.
The discussion above explains why particles can make the sky
appear hazy. The next
set of figures tries to explain how particles in the air can reduce
visibility.

The air is free of particles
in this first picture. You're
looking at a nearby mountain. The "side view" at left
explains that you are able to see the mountain because it reflects
sunlight back toward you (the green trees reflect green light, the
rocks and soil reflect brown light). The picture at right is what
you see
when you look at the mountain. We are ignoring any light
scattered by air molecules because the mountain is close by.
Now some particulates have
been
added to the air. They scatter sunlight, the scattered light is
white (it's highlighted in yellow in the picture at left for
emphasis). So now you still see the brown and green reflected
light but also some white scattered light. Some (fairly big)
spots of white light have been added to the picture at right.

More particles have been added
to
the air. That means there will be more scattered light.
We'll add even more particles to the air in the
next
picture.
Because there is more scattered light (more spots of white light in the
picture of the mountain) you have a harder time seeing the mountain.
Eventually all you see is the
scattered light. The mountain fades from view.
Here are some analogous situations that might help
understand how/why light scattered by particles in the air reduce
visibility.
Driving with a dirty windshield at night. Light from oncoming
traffic is scattered by dirt on the wind shield producing glare.
It is hard to see the other car and even harder to see a pedestrian or
a bicycle on the side of the road because of all the glare.
Trying to understand a student in the back of the room asking a
question when lots of students in the middle and front of the room are
talking. The students voice from the back of the room is "drowned
out" by all the noise coming from the rest of the front (note I'm not
implying there has been a lot of noise in the classroom, quite the
opposite so far this semester)
You might think that when the air is clean the visibility might
be unlimited. That isn't the case. Scattering
of sunlight by air molecules alone puts a limit on
visibility. The following figure tries to explain why this is so.
A nearby mountain would appear dark green or brown must as
it did in the above series of pictures.
You are
mostly seeing light reflected off the mountain with just a little light
scattered by air molecules.
As the mountain
gets further away you start seeing increasing amounts of blue light
(sunlight scattered by air molecules in between you and the
mountain) being added to the brown and green reflected light.
This is because there is more and more air between you and the
mountain. As
the mountain gets even
further away the amount of this blue light from the sky
increases.
Eventually the mountain gets so far away that you only see blue light
coming from the air and none of the light reflected by the mountain
itself.