Mon., Oct. 10, 2011
click here to download today's notes in
a more printer friendly format
Just about the best version of Stand By Me that
you'll ever hear from Playing
for
Change. There wasn't time but here's another Don't
Worry.
In addition to the usual Study Guide and the reviews there are a couple
of new items or events to help you prepare for this week's quiz.
First, in reponse to a student question, there's a list of pages
from the ClassNotes that cover Quiz #2 material. Also the class
Preceptor, Nicole Venn, is planning to conduct Open Study Hours from
6-7 pm at the Main Library (you can contact her by email
[nvenn@email.arizona.edu] or just meet on the ground floor of the
library near the elevators at 6 pm if you're interested).
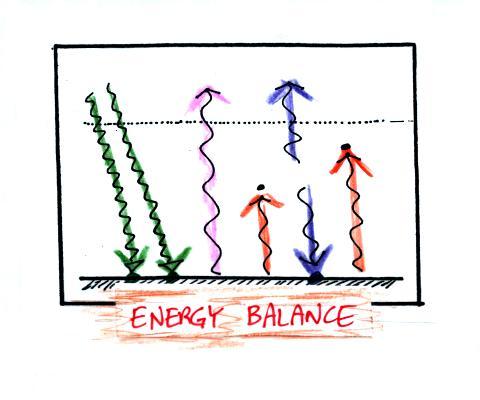
We spent a few minutes at the start of class reviewing material
covered in the 2nd half of class on Friday: a simplified representation
of energy balance on the earth with and without an atmosphere. I
won't repeat that material here, you can go back to the second half of
the online notes from Friday for that.
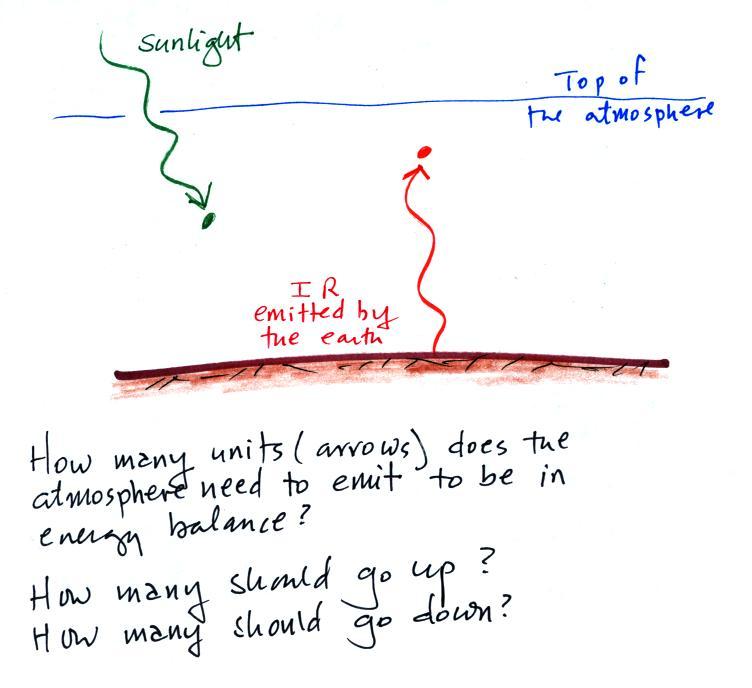
Here's a short question about energy balance to test your
understanding.
The atmosphere is absorbing 1 unit of incoming sunlight energy and
1 unit of IR energy coming from the ground. You basically need to
add some arrows to the picture and bring everything into energy
balance. A good place to start is to ask how many arrows the
atmosphere must emit. Then check for energy balance at the ground
and for balance between energy arriving at the earth and energy leaving
the earth and going out to space.
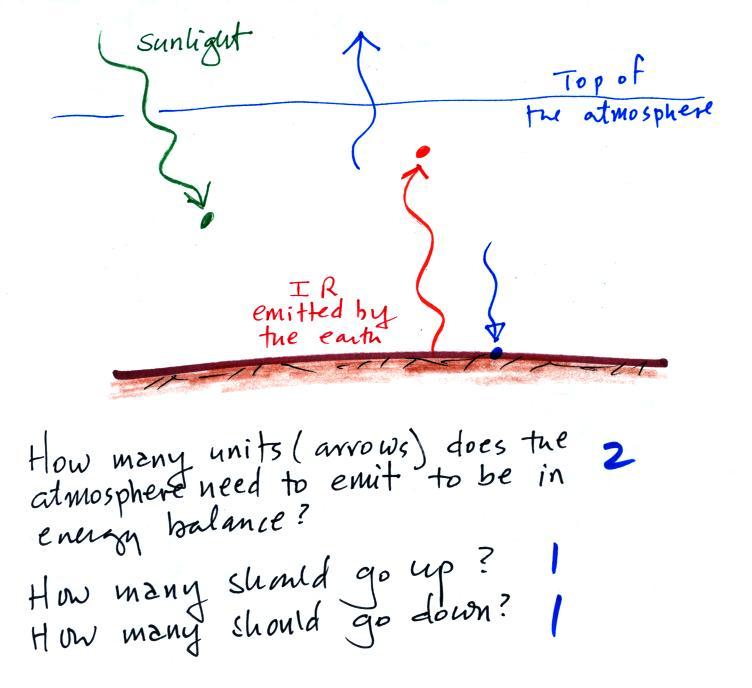
The atmosphere is absorbing two arrows and must emit 2 arrows to
be in energy balance. Send one of these down to the ground.
That will balance the 1 arrow of IR being emitted by the ground.
Draw the 2nd arrow pointing upward and going into space. Now we
have 1 arrow arriving at the top of the atmosphere from the sun and 1
arrow leaving the atmosphere and going back out into space.
In
our
simplified
explanation of the greenhouse effect we assumed that
100% of the sunlight arriving at the earth passed through the
atmosphere and got absorbed at the ground. We will now look at how
realistic that assumption is.
The bottom figure
above shows that on average (over the year and over the globe) only
about 50% of the incoming sunlight
makes it through the atmosphere and gets absorbed at the ground.
This is the only number in the figure you should try to remember.
About 20% of the incoming sunlight is absorbed by gases in the
atmosphere. Sunlight is a
mixture of UV, VIS, and IR light.
Ozone and oxygen will absorb a lot of the UV (though there isn't much
UV in sunlight) and greenhouse gases will absorb some of the IR
radiation in sunlight (Roughly half of sunlight is IR light).
The remaining 30% of the incoming sunlight is reflected or
scattered back into
space
(by the ground, clouds, even air molecules).
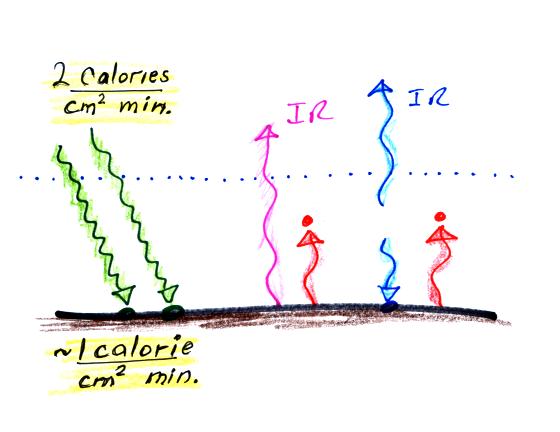
Student performing Experiment #3 will be measuring the amount of
sunlight energy arriving at the ground. About 2 calories pass
through a square centimeter per minute at the top of the
atmosphere. Since about half of this arrives at the ground on
average, students should expect to get an answer of about 1
calorie/cm2 min.
Next we
will look at our simplified version of radiative equilibrium and a more
realistic picture of the earth's energy budget.
In the top figure (the simplified
representation of energy balance) you should recognize the incoming
sunlight
(green),
IR emitted by the ground that passes through the atmosphere (pink or
purple), IR
radiation emitted by the ground that is absorbed by greenhouse gases in
the atmosphere (orange) and IR radiation emitted by the atmosphere
(dark blue).
The lower part of the figure is
pretty complicated. It
would be
difficult to start with this figure and find the greenhouse effect in
it. That's why we used a simplied version. Once you
understand the upper figure, you should be
able to find and understand the corresponding parts in the lower figure
(especially since I've tried to use the same colors for each of the
corresponding parts).
Some of the incoming sunlight (51 units in green) reaches the ground
and is absorbed. 19 units of sunlight are absorbed by gases in
the atmosphere. The 30 units of reflected sunlight weren't
included in the figure.
The ground emits a total of 117 units of IR light. Only 6 shine
through the atmosphere and go into space. The remaining 111 units
are absorbed by greenhouse gases. The atmosphere in turn emits
energy upward into space (64 units) and downward toward the ground (96
units). Why are the amounts different? One
reason might be that the lower atmosphere is warmer than the upper
atmosphere (warm objects emit more energy than cold objects).
Part of the explanation
is probably also that there is more air in the bottom of the atmosphere
(the air
is denser) than
near the top of
the atmosphere.
Notice that conduction,
convection, and latent heat energy transport (the 7 and 23 units on the
left side of the figure) are needed to bring the
overall energy budget into balance. The amount of energy transported by
conduction, convection, and latent heat is small compared to what is
transported in the form of EM radiation.
A couple more things to notice in the bottom figure (that I probably
didn't mention in class)
(i) The ground is actually receiving more energy from the
atmosphere (96 units) than it
gets from the sun (51 units)! Part of the reason for this is
that the sun just shines for part of the day. We receive energy
from the atmosphere 24 hours per day.
(ii) The ground emits more energy (117
units) than it gets from the sun (51 units). It is able to
achieve energy balance because it also gets energy from the
atmosphere (96 units).
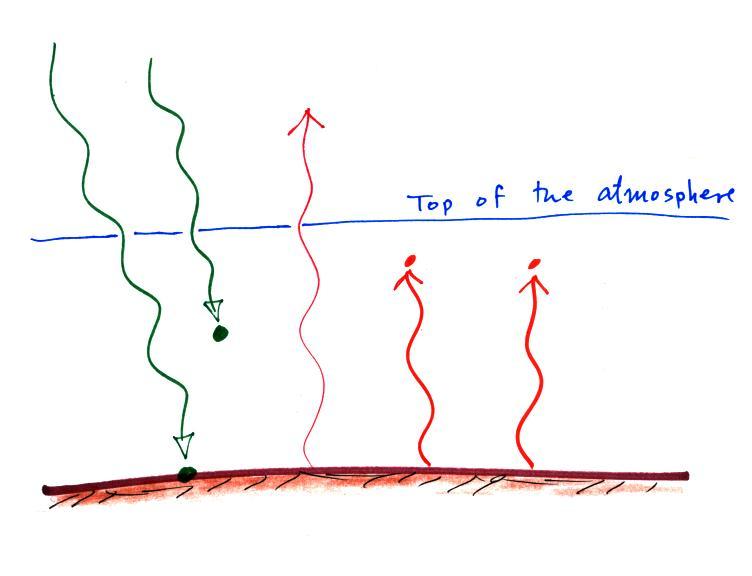
Here's another test your understanding style question. It's a
simplified but slightly more realistic version of energy balance on the
earth.
In this case 1 of the 2 incoming arrows of sunlight is absorbed in
the atmosphere instead of at the ground. The ground is still
emitting 3 arrows of IR light. Your task is to bring the picture
into energy balance. Again start with the atmosphere. How
many units does it need to emit. Then look at what is needed to
bring energy balance to the ground (which is now emitting 3 arrows and
only getting 1 from the sun). Look also at the numbers of units
of energy arriving at the top of the atmosphere and leaving the
atmosphere.
The atmosphere needs to emit 3 arrows of IR light. 1
goes upward and into space, the other two go downward and get absorbed
by the ground.
Next we used our simplified representation of the greenhouse
effect to understand the effects of clouds on daytime high and
nighttime low temperatures. The following can be found on pps.
72a & 72b in the ClassNotes (I've rearranged things slightly to
make it clearer)
Here's the simplified picture of
radiative equilibrium again (you're probably getting pretty tired of
seeing this). You should be able to say something
about every arrow in the picture. The
two pictures below show what happens at night
when you remove
the
two green rays of incoming sunlight.

The picture on the left shows a
clear night. The ground is losing
3
arrows of energy and getting one back from the atmosphere. That's
a
net loss of 2 arrows. The ground cools rapidly and gets cold
during
the night.
A cloudy night is shown at right. Notice the effect of the
clouds.
Clouds are good absorbers
of infrared
radiation. If we could see IR light,
clouds would appear black, very different from what we are used
to (because clouds also emit IR light, if we could see IR light the
clouds might also
glow). Now none of
the IR radiation emitted by the ground passes through the atmosphere
into space. It is all absorbed either by greenhouse gases or by
the
clouds. Because the clouds and atmosphere are now absorbing 3
units of
radiation they must emit 3 units: 1 goes upward into space, the other 2
downward to the ground. There is now a net loss at the ground of
only
1 arrow.
The ground won't cool as quickly and won't get as cold on a cloudy
night as it does on a clear night. That makes for nice early
morning bicycle rides this time of the year.
The next two figures compare clear and cloudy days.

Clouds are good reflectors
of visible
light (we see visible light and clouds appear white). The effect
of this is to
reduce the amount of sunlight energy reaching the ground in the right
picture. With less sunlight being absorbed at the ground, the
ground
doesn't need to get as warm to be in energy balance.
It is generally cooler during the day on a cloudy day than on a
clear
day.
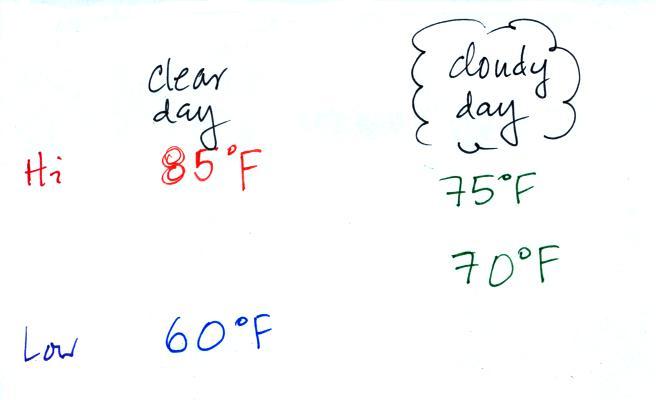
Clouds raise the nighttime minimum temperature and lower the
daytime
maximum temperature. Here are some typical daytime high and
nighttime
low temperature values on clear and cloudy days for this time of the
year.
We'll use
our simplified representation of radiative equilibrium to understand
enhancement of the greenhouse effect and global warming.
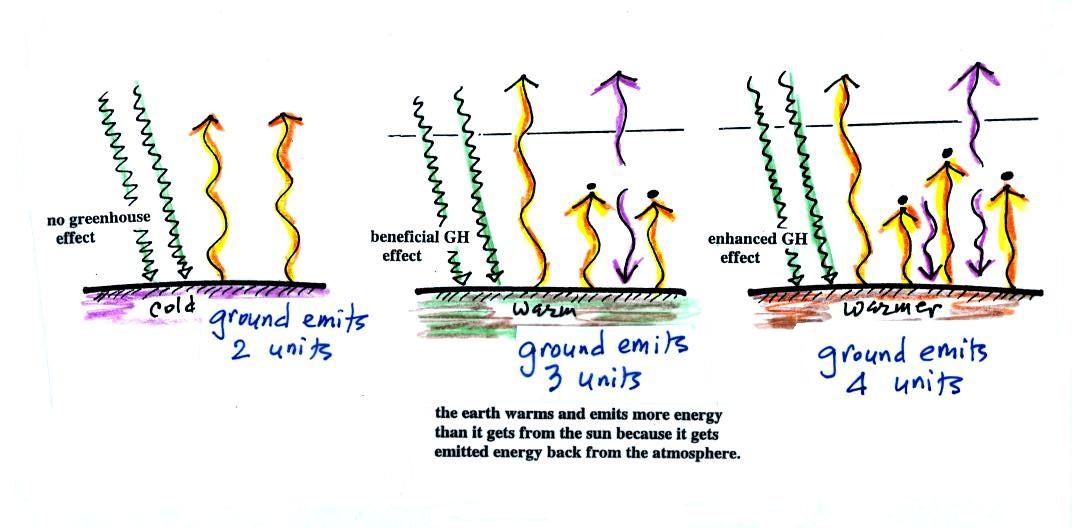
The figure (p. 72c in the
photocopied Class Notes) on the
left
shows
energy balance on the earth
without
an atmosphere (or with an atmosphere that doesn't contain greenhouse
gases). The ground achieves energy balance by emitting only 2
units of energy to balance out what it is getting from the sun.
The ground wouldn't need to be
very warm to do this.
If you add an atmosphere and greenhouse gases, the atmosphere will
begin to absorb some of the outgoing IR radiation. The atmosphere
will also begin to emit IR radiation, upward into space and downard
toward the ground. After a period of adjustment you end up with a
new energy balance. The ground is warmer and is now emitting 3
units of energy even though it is only getting 2 units from the
sun. It can do this because it gets a unit of energy from the
atmosphere.
In the right figure the concentration of greenhouse gases has
increased
even more (due to human activities). The earth would find a new
energy balance. In this case the ground would be warmer and would
be emitting 4 units of energy, but still only getting 2 units from the
sun. With more greenhouse gases, the atmosphere is now able to
absorb 3
units of the IR emitted by the ground. The atmosphere sends 2
back to the ground and 1 up into space.
The next figure shows a common misconception about the cause of
global
warming.
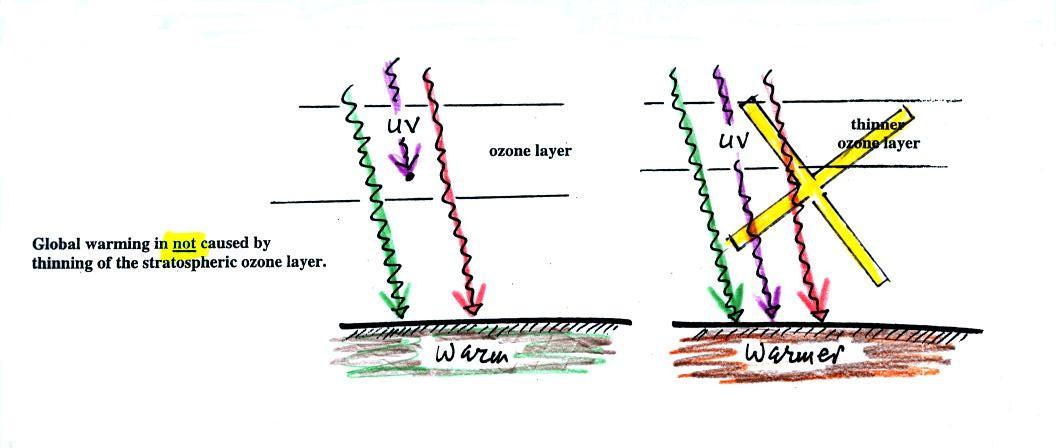
Many people know that sunlight
contains UV light and that
the ozone
absorbs much of this dangerous type of high energy radiation.
People also know that release of chemicals such as CFCs are destroying
stratospheric ozone and letting some of this UV light reach the
ground. That is all
correct.
They then conclude that it is
this additional UV energy reaching the ground that is causing the globe
to warm. This
is not correct. There isn't much UV light in sunlight in
the
first place and the small amount of additional UV light reaching the
ground won't be enough to cause global warming. It will cause
cataracts and skin cancer and those kinds of problems but not global
warming.