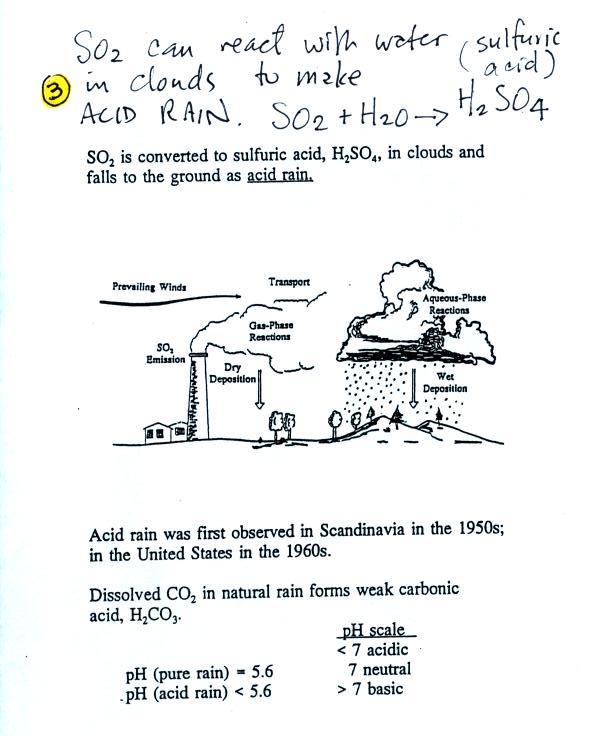
Clouds and precipitation are the
best way of cleaning pollutants from the air.
Before
beginning this section, you should familiarize yourself with the
concept of light scattering by looking at the Light Scattering Activity.
Particulates
can affect visibility and can make the sky appear hazy. To
understand this we need to look at how air molecules and particles
scatter sunlight.
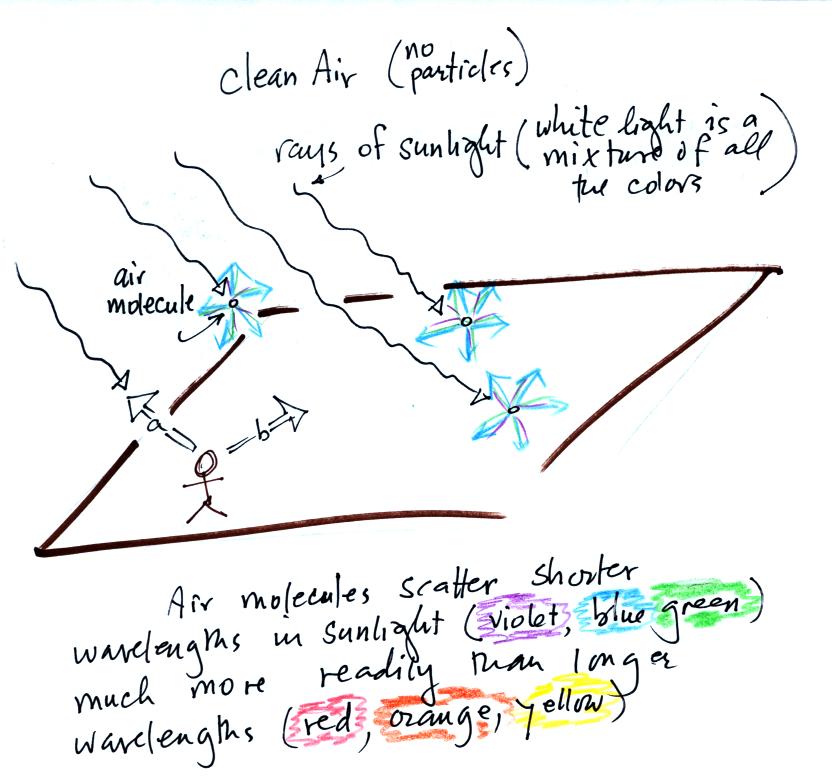
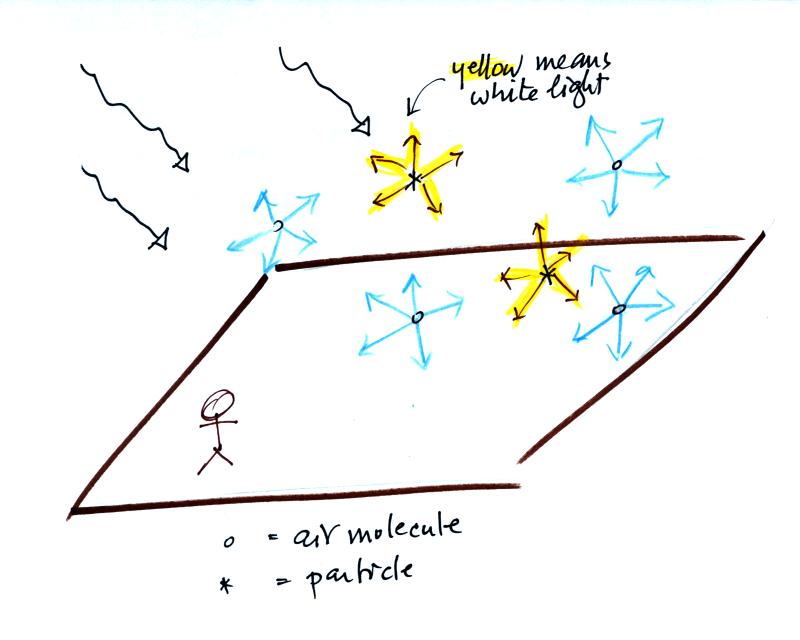
Rays of sunlight are passing
through clean air above. You wouldn't ordinarily be able to see
the sunlight unless you looked back along one of the rays (a) in the
figure, i.e. back toward the sun. You'd see the sun in that case
(at least up until you caused some serious damage to your eyes).
If you look away
from the sun toward the sky (b) you see blue light. This is light
that is being scattered by air molecules.
Sunlight is white light, which tells you it is a mixture of all the
colors. Because air molecules are small (relative to the
wavelength of visible light) they scatter shorter wavelengths more
readily than longer wavelengths. When you look away from the sun
and toward the sky you see this scattered light, it has a deep blue
color. This is basically why the sky is blue. If the earth
didn't have an atmosphere (or if air molecules didn't scatter light)
the sky would be black.
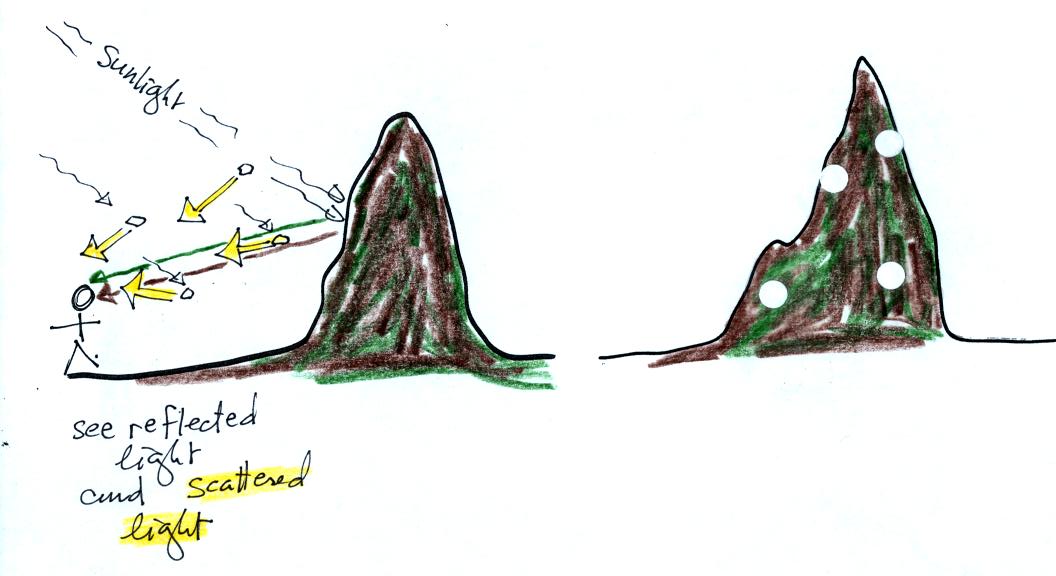
Scattering
of sunlight by air molecules turns distant
mountains blue
and eventually makes them fade from view.
A nearby mountain might appear dark green or brown. You are
mostly seeing light reflected off the mountain. As the mountain
gets further away you start seeing increasing amounts of blue light
(sunlight scattered by air molecules in between you and the
mountain) being added to and mixed in with the brown and green
reflected light. As
the mountain gets even
further the amount of this blue light from the sky increases.
Eventually the mountain gets so far away that you only see blue sky
light and the light reflected by the mountain itself becomes so weak it
can't be seen.
We've added some particles to the
air in the picture above. Particles also scatter light.
But because the particle size is about equal to or somewhat greater
than the wavelength of visible light the particles scatter all the
colors equally. The light scattered by particles is white.
This is basically why clouds are white.
As the amount of particulate matter in the air increases the color
of
the sky changes from deep blue to whitish blue. The higher the
particle concentration, the whiter the sky becomes.
Have a look at the color of the sky before and after a rainstorm.
Before the storm, the air will be full of particulate pollution and
will appear whitish blue. After the storm, after the rain has
removed a lot of the pollutants, the sky often has a much deeper blue
color.
The next
set of figures tries to explain how particles in the air can affect
visibility.