Friday Feb. 17, 2006
Quiz 1 was returned in class. Be sure to carefully check the
grading and make sure the points missed were added correctly.
The Reading Assignments link has
been updated.
If you were not able to attend class on Friday here are some special instructions for you.
It is easy
to lose sight of our overall objectives in Chapter 2 because of all the
details. That's the reason for this introduction. Some of
the major topics in Chapter 2 are mentioned below (found on pps 43-44
in the photocopied class notes).
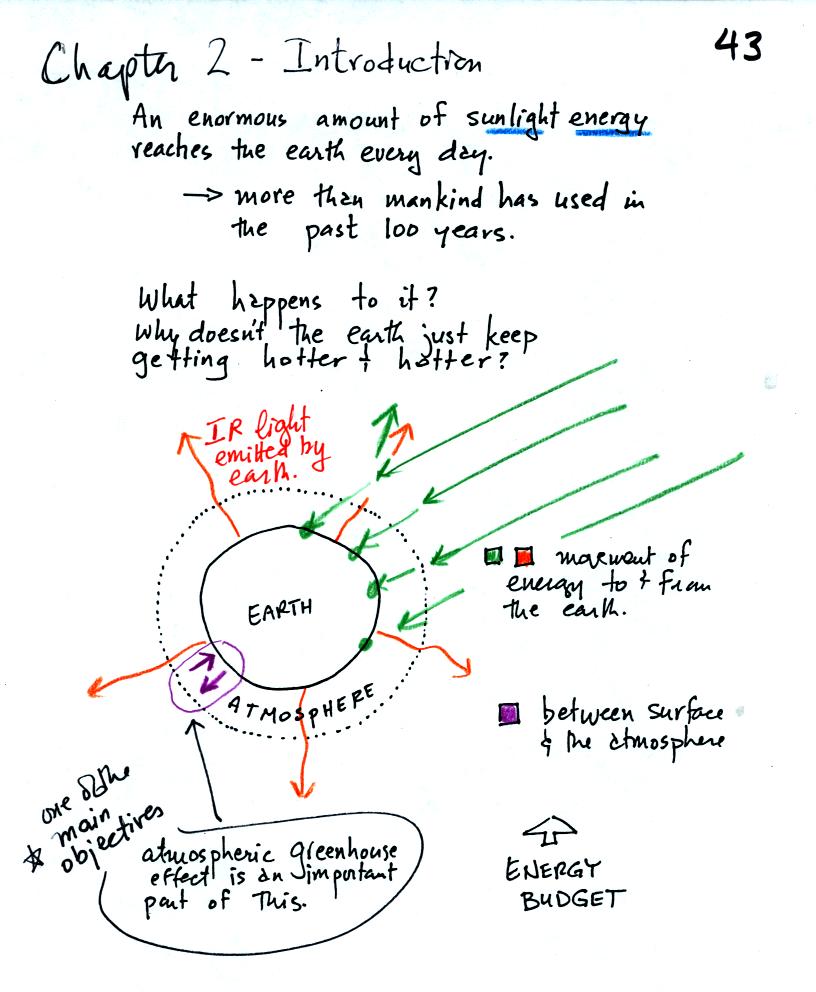
An enormous amount of sunlight energy reaches the earth
everyday.
We will learn how it is possible for this form of energy to travel
through empty space. The next figure shows that sunlight consists
of a
little Ultraviolet light, and roughly equal amounts of Visible
light (44%), and Infrared light
(48%) [the remaining 1% is composed of microwaves, radiowaves and
things like that]. With all of this energy arriving at and being
absorbed by the earth, what keeps the earth from getting hotter and
hotter? The answer is that the earth also sends energy back into
space (an invisible form of energy - infrared light). A balance
between incoming and outgoing energy is achieved and the earth's annual
average temperature remains constant.
We will also look closely at energy transport between the earth's
surface and the atmosphere. That is where the atmospheric
greenhouse operates. That will be a major goal in Chapter 2 - to
understand the atmospheric greenhouse effect.
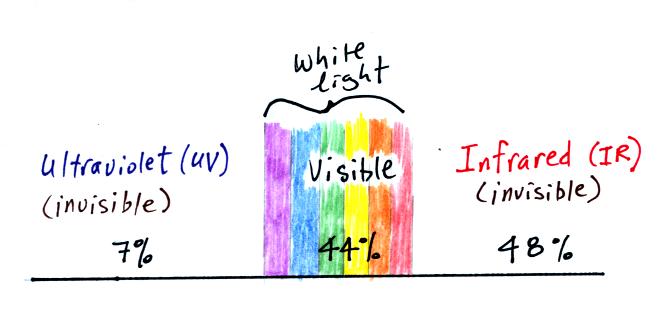
We use color to distinquish between different types of visible
light. Ultraviolet light has been divided into 3 groups according
to wavelength:

UV-C is the most energetic form of UV light and potentially the most
dangerous. Fortunately it is absorbed by the ozone layer in the
stratosphere. UV-B causes skin cancer but is also used by the
body to produce vitamin D. UV-A damages the skin.
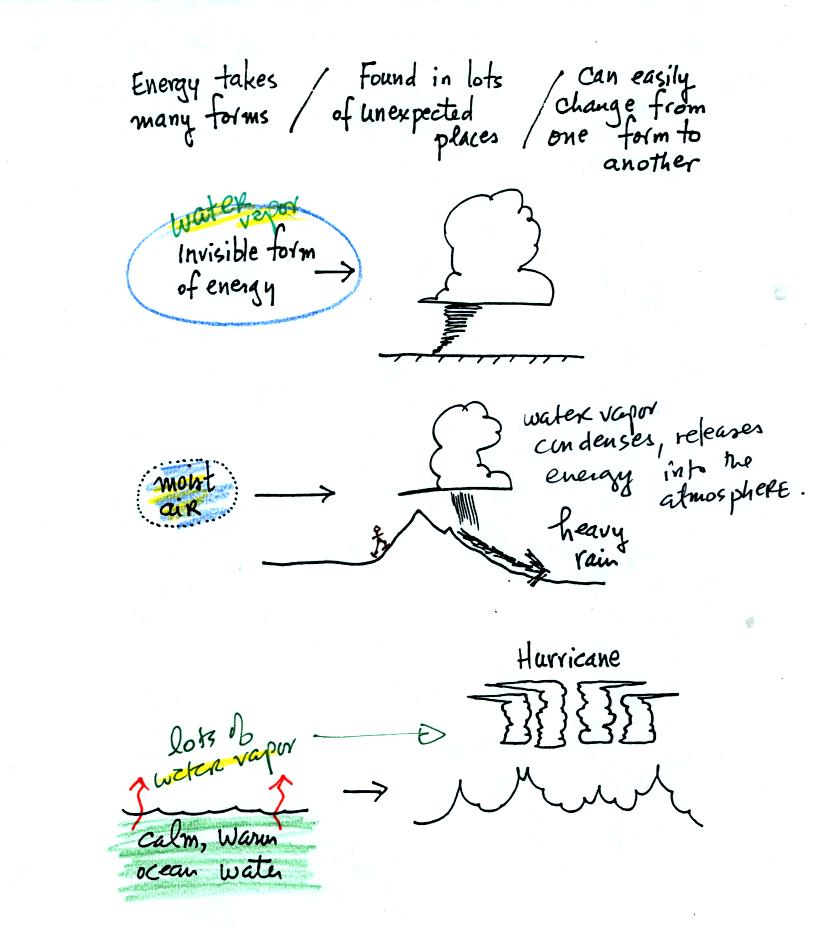
Water vapor is a particularly important form of invisible
energy.
When water vapor condenses to produce the water droplets in a
cloud, an enormous amount of energy is released into the atmosphere.
It is hard to visualize or appreciate energy released into the
atmosphere during condensation. You can imagine the work that you
would do carrying a gallon of water
(8 pounds) from Tucson to the top of Mt. Lemmon, however. To
accomplish
the same thing Mother Nature must first evaporate the water and (if my
calculations are correct) that requires about 100 times the energy that
you would use to carry the 8 pounds of water to the summit of Mt.
Lemmon. And Mother Nature transports a lot more than just a
single gallon.

Kinetic energy is energy of motion. Latent heat energy
is an unappreciated form of energy.
The four energy transport
processes are listed at the bottom of the page above. By far the
most important process is electromagnetic radiation. This is the
only process that can transport energy through empty space.
Electromagnetic radiation is also responsible for about 80% of the
energy transport between the ground and atmosphere. You might be
surprised to learn that latent heat is the second most important
transport process.
One of the main objectives in Chapter 2 to understand the
greenhouse
effect.
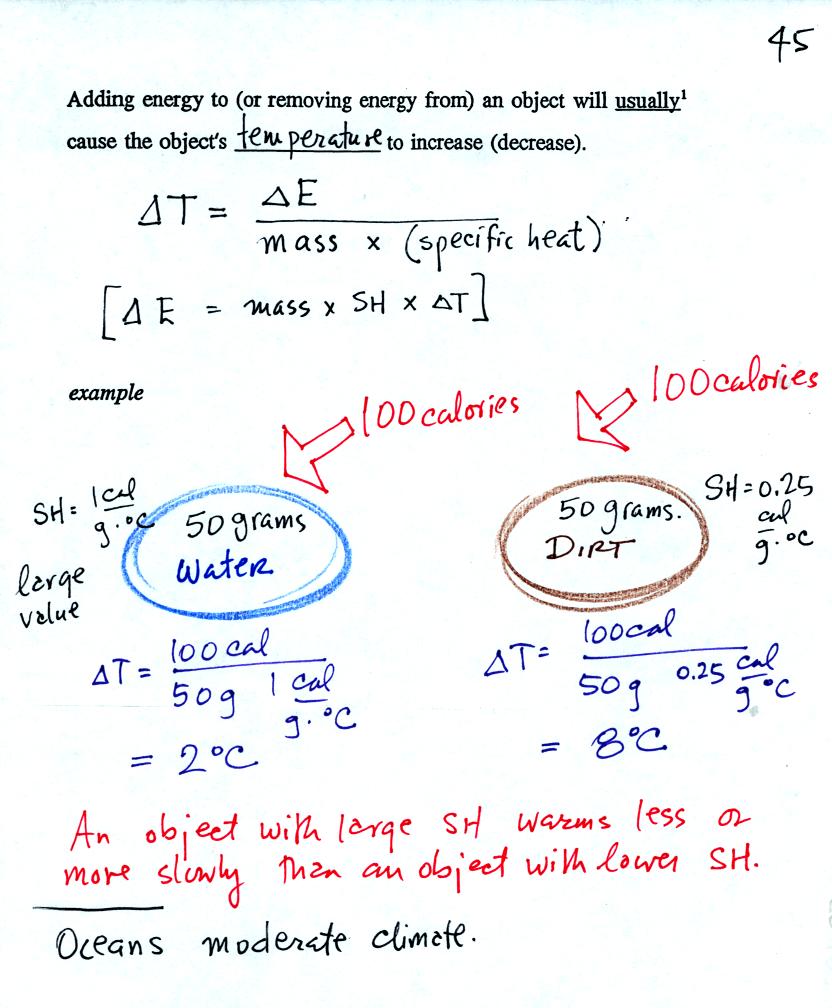
Here's the relationship between energy added to a material and the
temperature change that follows. We will look at an important
example to try to better understand the effect of specific heat.
We add equal amounts of energy (note calories are units of energy) to
equal masses of water and soil. Water has a relatively high
specific heat and warms less than the soil. These two materials
were used in the example because the surface of the earth is made up of
water (oceans) and soil. Oceans moderate the climate. It is
hard to warm the ocean in the summer and hard to cool the ocean in the
winter. A city near an ocean will have less annual swing in
temperature than a city located in the middle of land mass.

Two cities are located at the same latitude and altitude. One is
on a coast, the other is inland. The city on the coast has cooler
summers and warmer winters than the city further inland. The
annual range of temperature (difference between summer and winter) is
30 F on the coast and 60 F further inland.
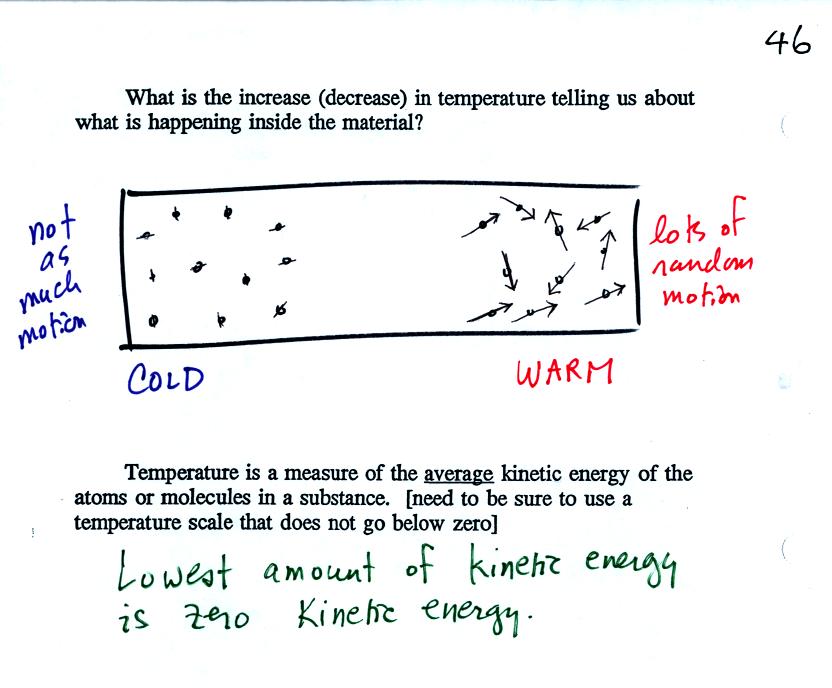
Temperature provides a measure of the average kinetic of the atoms or
molecules in a material. The Kelvin temperature scale does not go
below zero.
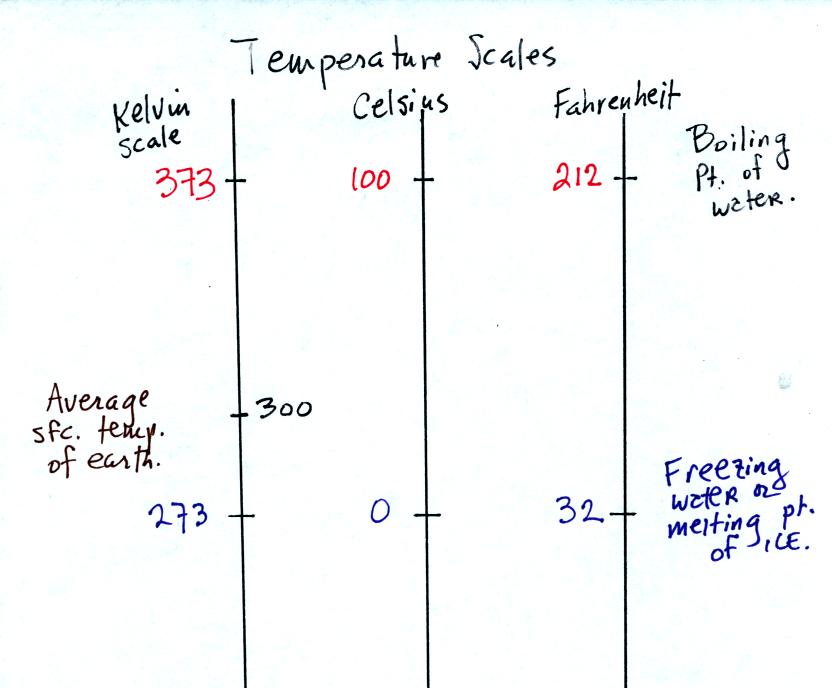
You should remember the temperatures of the boiling point and freezing
point of water on the Fahrenheit, Celsius, and Kelvin scales. A
good global annual average surface temperature for the earth is 300
K.
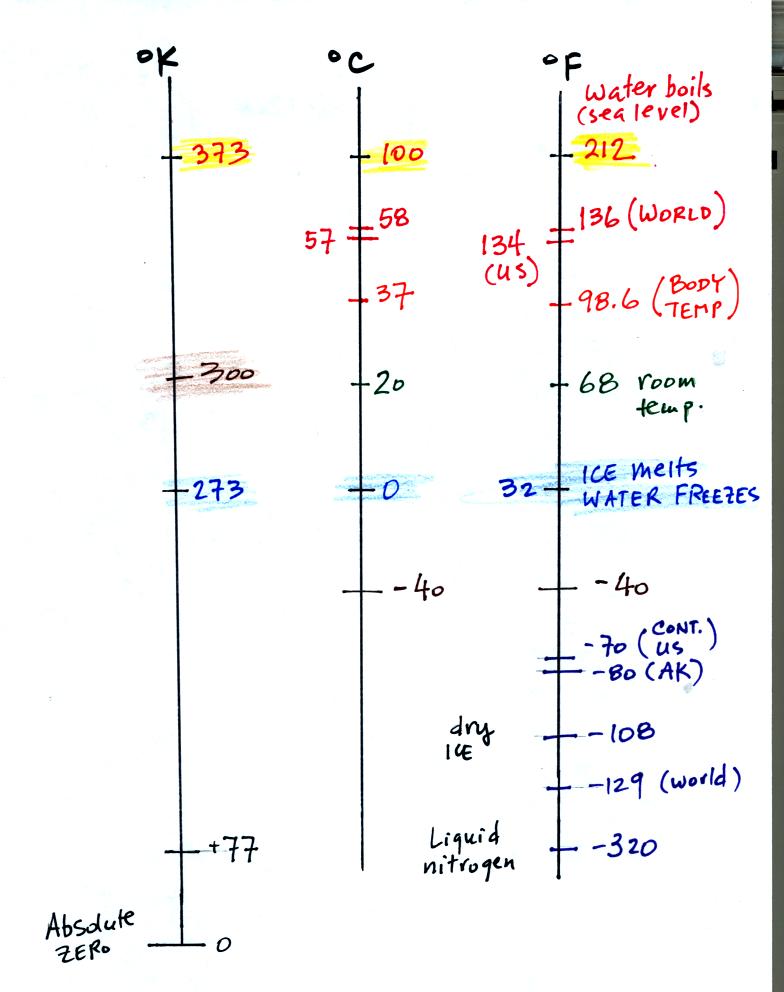
The world high temperature record was set in Libya, the US record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than near or
surrounded by ocean
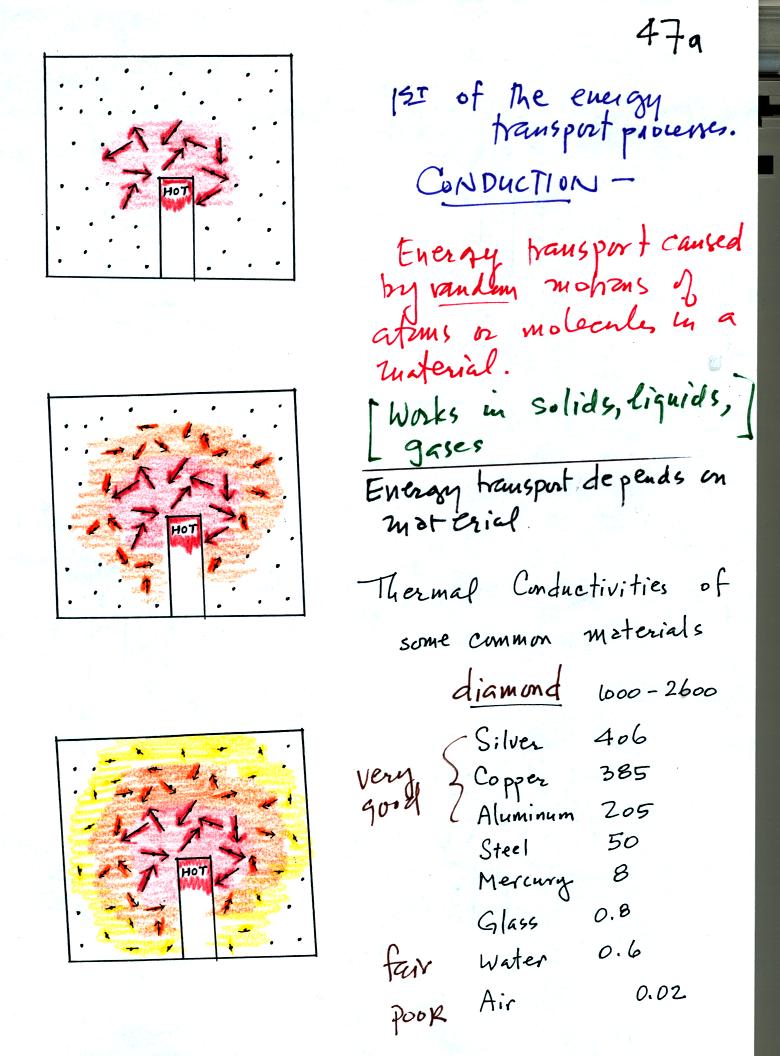
The figure above illustrates energy transport by conduction. A
hot object is stuck in the middle of some material (gas, liquid, or
solid). In the first picture the random motions of the atoms or
molecules near the object have caused them to collide with and pick up
energy from the object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms. In the middle picture the energetic molecules have
collided with some of their neighbors and shared energy with
them. The neighbor molecules have gained energy though they don't
have as much energy as the molecules next to the hot object. In
the third picture molecules further from the object now have gained
some energy. The random motions and collisions between molecules
is carrying energy from the hot object out into the material.
The rate of energy transport depends on the material. Thermal
conductivities of some materials are listed above. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors. Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.











