Monday Feb. 20, 2006
A limited number of students (not in class on Friday) were able to pick
up their quiz because they had gone to the Friday
lecture notes and followed the special instructions given there.
There are a couple of Expt. 1 reports (R. Cahan, J. Knorpel) that
haven't been picked up yet (also a few people that haven't returned
their Expt. 1 materials). You are allowed to revise your original
report. The revised reports are due next Monday. Please
return the original report when you turn in a revised report. The
Experiment #2 reports are also due next Monday. More Expt. 2
materials are becoming available. If you haven't checked out
Expt. 2 materials you can do so in class this week. You will be
given some extra time to do the experiment and prepare your report.
We
covered, conduction, the first of four energy transport processes on
Friday. In conduction energy is transported by the random motions
of the atoms or molecules in a material. The rate at which energy
is transported depends on (1) the material (air is poor conductor,
water is better, metals are good conductors) and (2) the temperature
difference or gradient between the energy source and the material (more
energy transported or more rapid rate of energy transport when the
temperature difference is large).
In many respects conduction of energy is like the diffusion of smell in
a classroom. Imagine opening a bottle containing concentrated
acetic acid. Acetic acid gives vinegar its distinctive smell.

With time the smell of the acetic acid would diffuse or spread
throughout a classroom. People in the back of the room might only
detect a faint odor of vinegar. In the middle of the room the
smell would be stronger. The concentration might be high enough
in the front of the room might be high enough to be hazardous (the main
reason this demonstration wasn't done).
What if you wanted to clear to room of the odor. You would first
close the bottle and then open the doors and eventually all of the
vinegar smell would diffuse out of the room. To speed things up
you might bring in some fans and force the air to circulate through the
room more quickly. The same kind of idea can be applied to energy
transport.

Convection is a second way of transporting energy. Convection
involves more organized motion of atoms or molecules in a liquid or gas
(but not in a solid, the atoms or molecules aren't able to move freely
enough).
In the top picture above the air surrounding a hot object has been
heated by conduction. Then a fan is switched on and the warm air moves
off to the right. Cooler air moves in and surrounds the hot
object and the cycle can repeat itself. This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly.
Note, in the bottom left figure, that the hot air is also low density
air. It actually isn't necessary to blow on the hot object, the
warm air will rise by itself. Energy is being transported away
from the hot object. This is called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands (as it
moves into lower pressure surroundings) and cools. If the air is
moist, clouds can form.
Note the example at right is also free convection. The sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.

Metals are better conductors than wood. If you touch a piece of
70 F metal it will feel colder than a piece of 70 F wood. A piece
of 70 F diamong would feel even colder because it is a better conductor
than metal. Our perception of cold is more an indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
Touching a piece of ice also feels colder even though ice is not an
especially good conductor. The cold feeling tells us that our
hand is losing a lot of energy. IN this case the high rate of
energy loss is due to the large temperature differrence between our
hand and the ice rather than the thermal conductivity of the ice.
If you go outside on a 40 F day (calm winds) you will feel cold; your
body is losing energy to the colder surroundings. A thermometer
behaves differently. It actually cools to the temperature of the
surroundings. Once there it won't lose any additional energy.
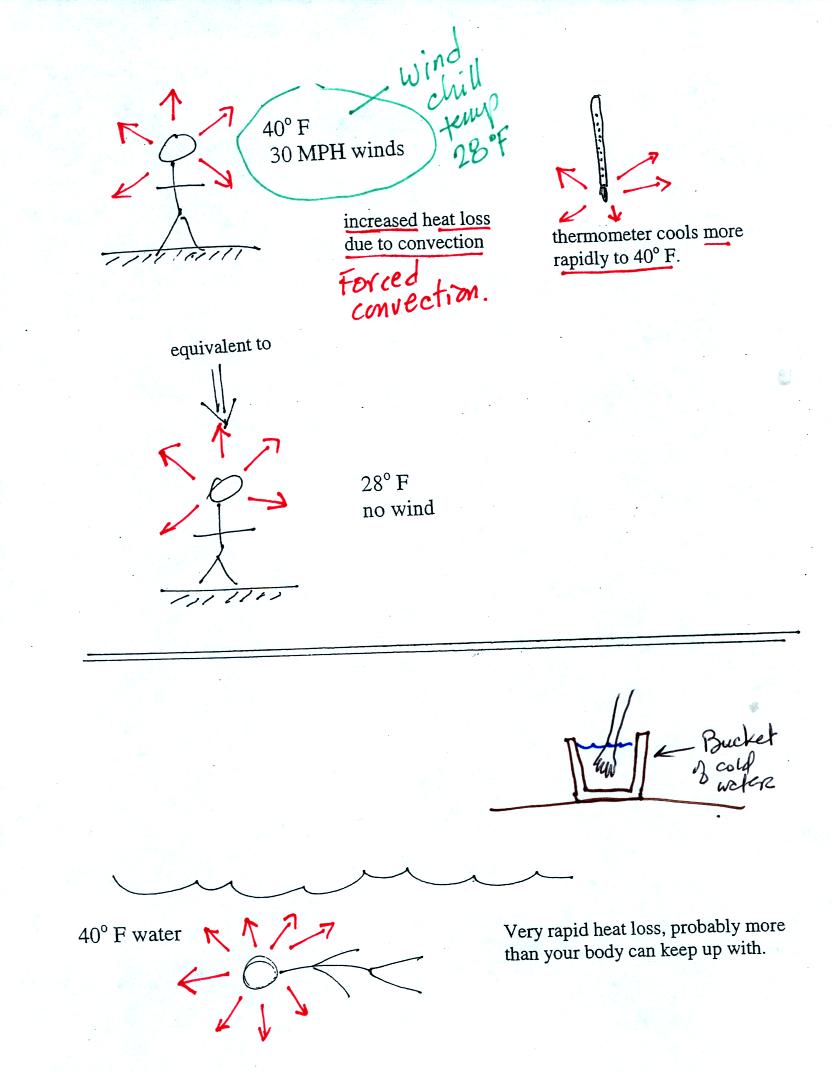
If you go outside on a 40 F day with 30 MPH winds your body will lose
energy at a more rapid rate. It will feel colder than a 40 F day
with calm winds. Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a calm 28
F day. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F. The thermometer will again cool to the
temperature of its surroundings. ON a windy day it will cool more
quickly, but once it ends up at 40 F it won't cool any further. The
thermometer would measure 40 F on both the calm and the windy day.
Water is a much better conductor than air. If you fall into 40 F
water your body will lose energy at a high enough rate that your
metabolism might not be able to keep up with it.
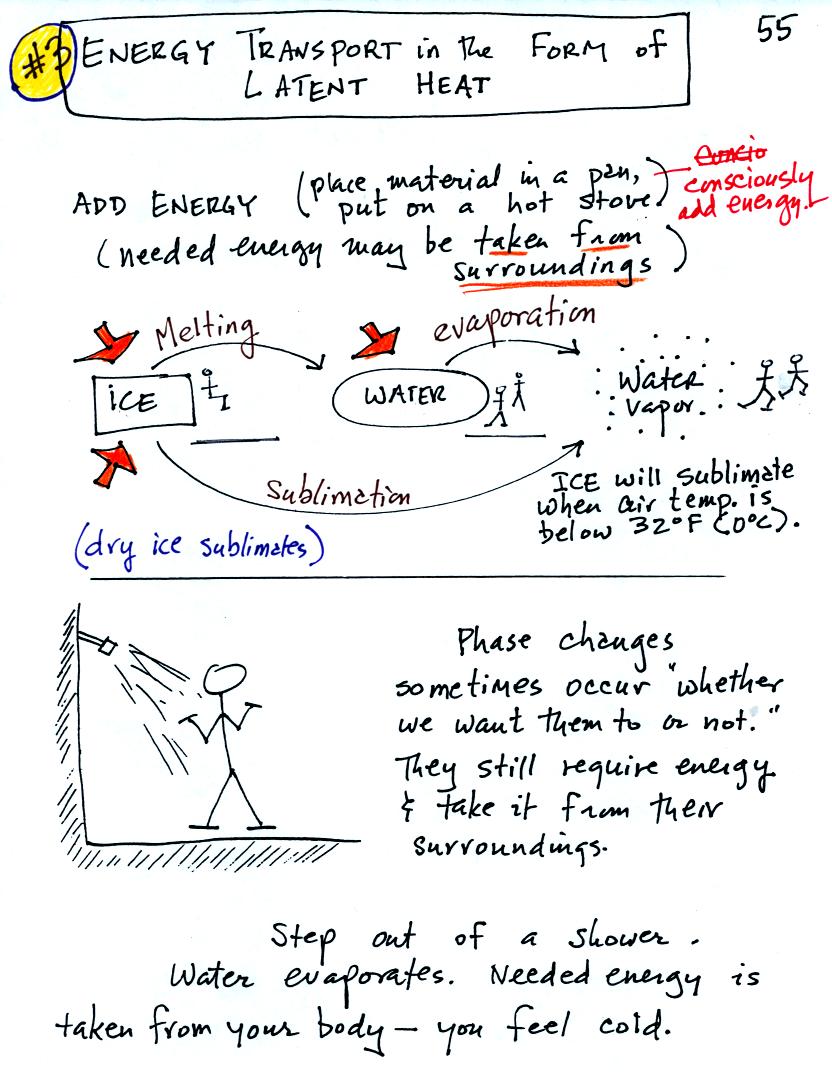
There are enormous amounts of "hidden" latent heat energy in water and
water vapor. This energy can appear when water vapor condenses or
water freezes.
A solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change. In
each case energy must be added to the material changing phase.
You can consciously supply the energy or the needed energy will be
taken from the surroundings (causing the surroundings to cool).
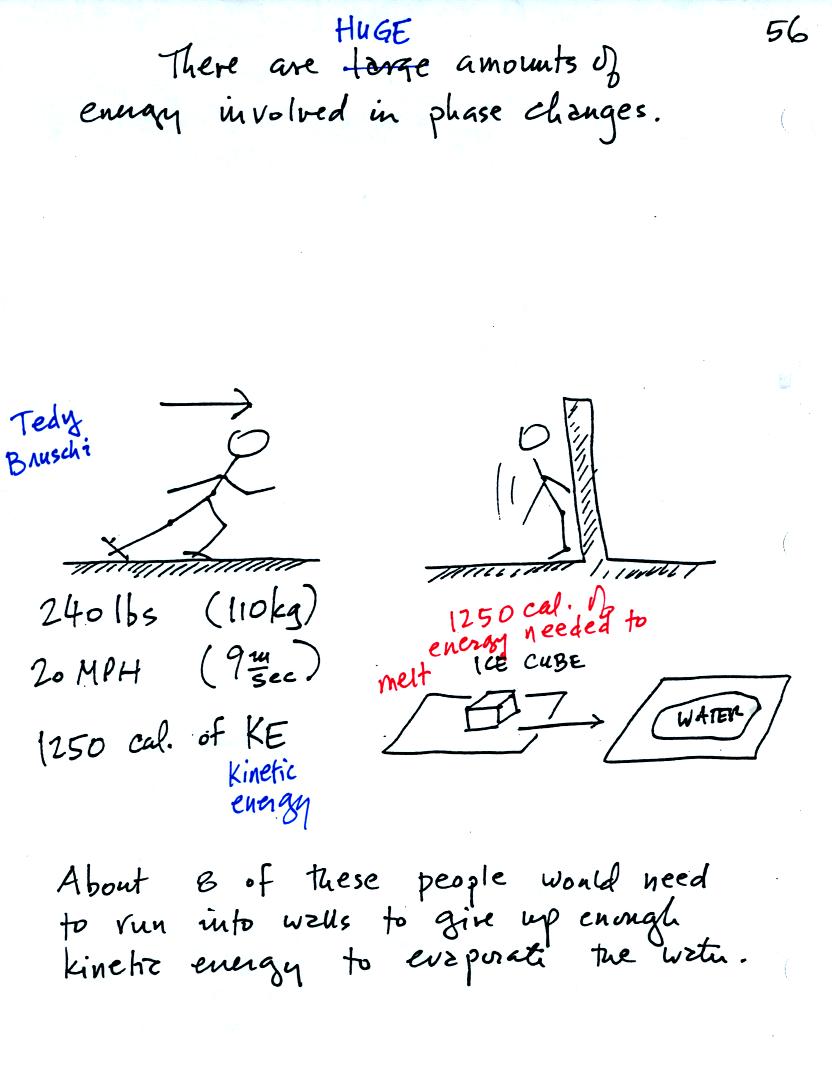
A 240 pound man (or woman) running at 20 MPH has just enough energy to
be able to melt an ordinary ice cube. It would take 8 people to
evaporate the resulting water.

You can consciously remove energy from water vapor to make it condense
or from water to cause it to free. Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm).
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more rapidly
Here's a
school kid analogy. A child sitting in their chair is analogous
to the atoms or molecules bonded together in a solid, a child
walking around in a classroom is like the atoms or molecules that are
able to move more freely in a liquid, and children running around
outside on a playground are more like the atoms or molecules in a gas.
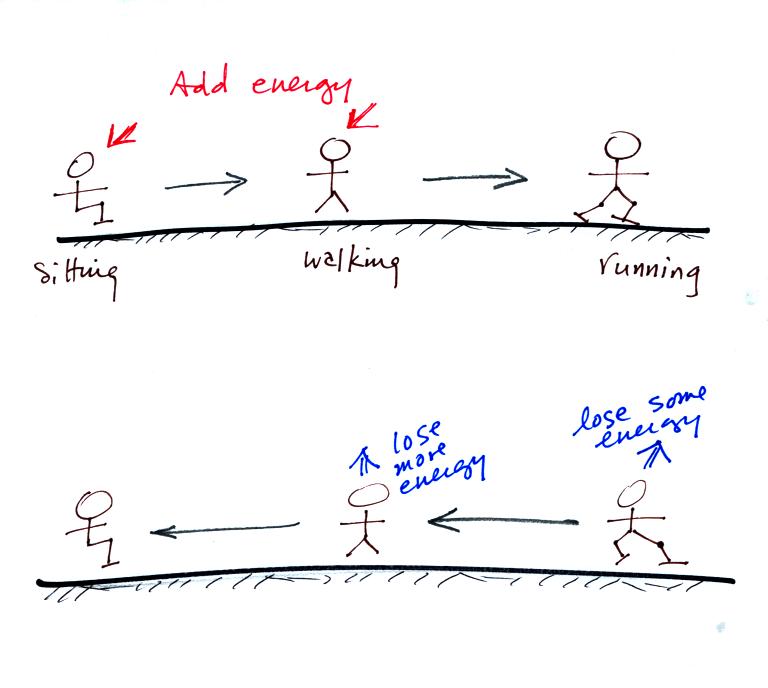
You need to "add energy" to get a kid out of its chair and running
around outside on the playground.
Then the difficult part, getting the child to get rid of some of that
energy before coming back into and sitting down in the classroom.
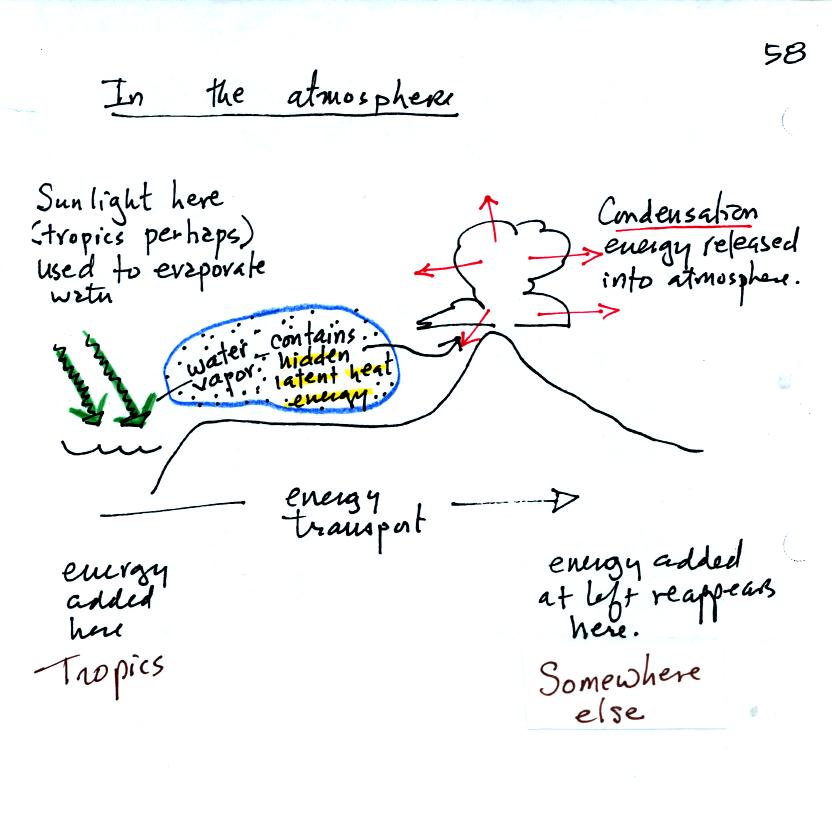
Here we put everything together. Starting at left in the
tropics where there is often an abundance or excess of energy, sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy. This hidden energy
reappears when something causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics can be transported to and
reappear in the atmosphere in a place like Tucson.








