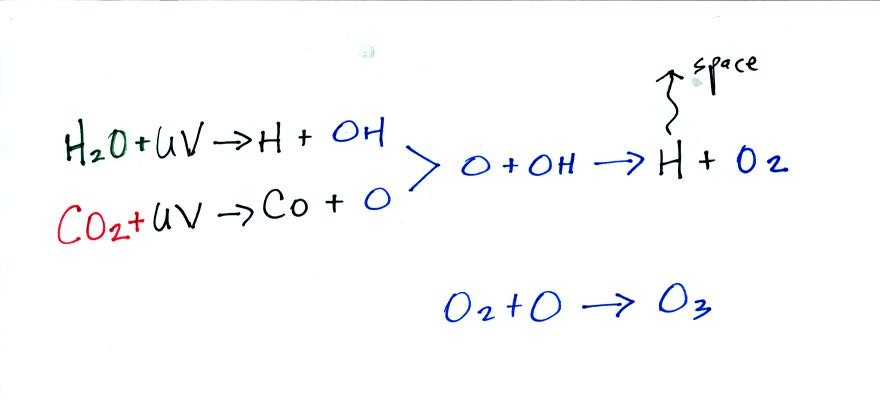Wednesday, Jan. 11, 2006
First day of class.
We first discussed the Course Information
handout. You should try to purchase a copy of the photocopied
notes right away as we will be using some of them in class on Friday.
Not shown on the course information sheet are the names of the two
teaching assistants (TAs) assigned to this class. Jason Criscio
will be available Monday from 3-5 pm in PAS 526. You can contact
him at criscio@atmo.arizona.edu. Theresa Foley is the name of the
other TA. She has not yet told me what her office hours will be.
Next we looked at the Writing Requirements
handout. You should be thinking about which of the experiments
(or book or scientific paper reports) you would like to do so that you
can sign up in class on Friday. Distribution of the materials for
the first experiment will begin Wednesday next week (Jan. 18).
This first of the 1S1P assignments has been
made. Reports are due on Monday Jan. 30.
We spend the last few minutes of class listing the most abundant and
important trace gases in the earth's atmosphere. This is also covered
at the beginning of Chapter 1 in the textbook (see the Reading Assignments link on the class
web page).
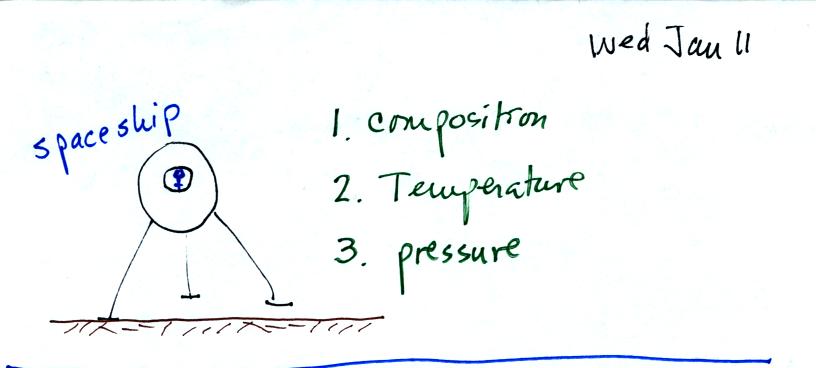
Imagine that a space vehicle has just landed in Tucson.
Before stepping outside the "person" inside would want to know
something about the composition, temperature, and pressure of the air
outside. The first part of Chapter 1 in the course textbook is
concerned with these topics.
We spent the last few minutes of class listing the most abundant and
some of the most important trace gases in the earth's atmosphere.
Here's the list:
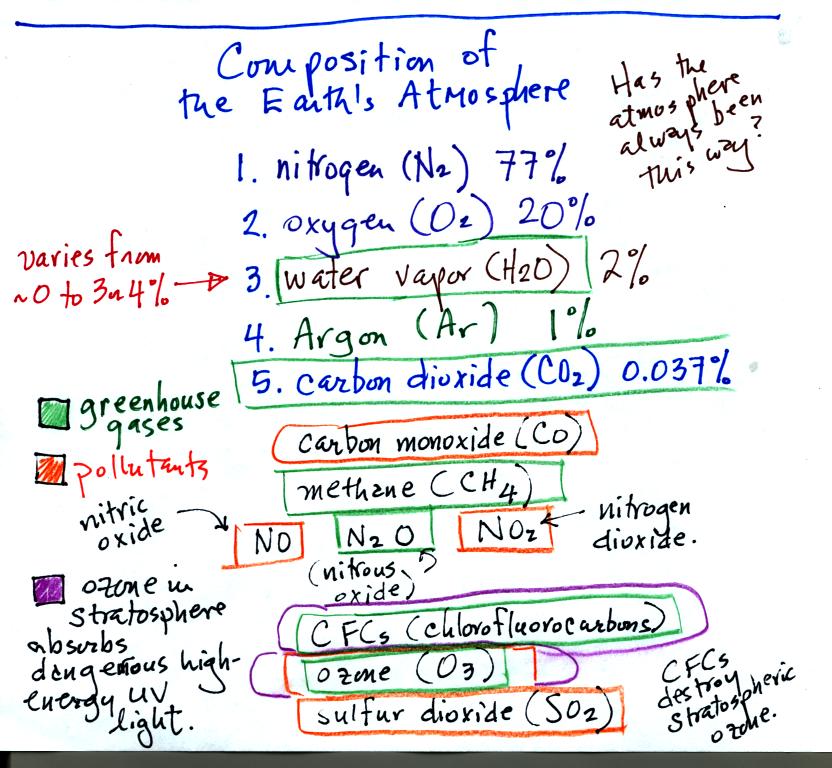
Nitrogen and oxygen are the most two abundant gases in the
atmosphere. Water vapor and argon occupy 3rd and 4th place.
The variable concentration of water vapor means it is sometimes more
abundant & sometimes less abundant than argon. Note water
vapor is an invisible gas. When you see steam, fog, or a cloud
you are seeing small drops of liquid water or small ice crystals not
water vapor.
Water vapor, carbon dioxide, methane, nitrous oxide,
chlorofluorocarbons, and ozone are greenhouse gases. We will
cover the greenhouse effect in more detail when we get to Chapter
2. The "natural" greenhouse effect has a beneficial role on the
earth. Without the greenhouse effect average surface temperatures
on the earth would be much colder than they are now. Atmospheric
concentrations of many greenhouse gases are increasing however.
This could enhance or strengthen the greenhouse effect and cause global
warming which could have many detrimental effects.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the main air pollutants. We'll discuss some
of them in more detail on Friday and next Wednesday.
Ozone in the stratosphere absorbs dangerous high energy ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
The
following information was not covered in class. I will bring
attention to it briefly in class on Friday.
Information on one of the atmospheric constituents in the list above is
given in the daily weather report. Do you know which one it would
be? The answer is water vapor. The weather person doesn't
report the percentage water vapor concentration however. They
will usually report the relative humidity. Occasionally they'll
give the dew point temperature. As we'll see later in the
semester, dew point temperature is a much better measure of water vapor
concentration than relative humidity. Here's a rough idea of how
dew point temperature relates to atmospheric water vapor concentration:
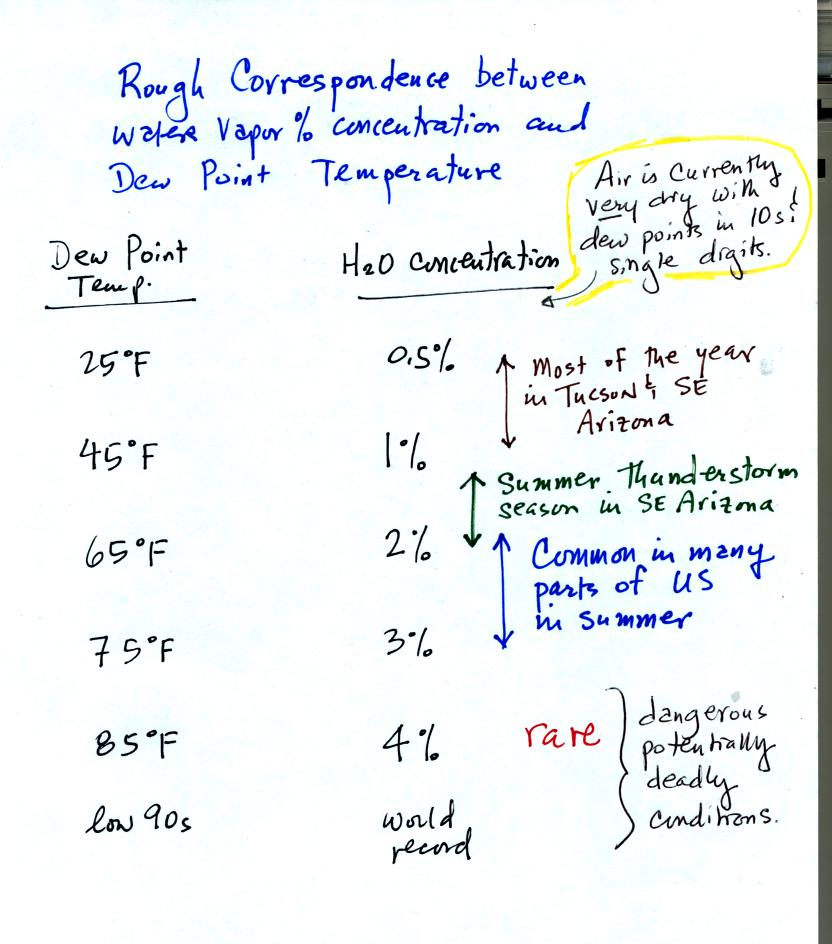
You can think of dew point as just being a number. When the value
is low the air doesn't contain much moisture. The higher the dew
point value, the more water vapor in the air. Dew points are
currently in the teens and single digits in SE Arizona, the air is very
dry.
The summer thunderstorm season (summer monsoon) begins officially in
Tucson when the daily average dew point temperature is 54o F
or above
for three days in a row. Dew points will remain in the upper 50s
and lower 60s throughout most of the summer thunderstorm season.
You'll find a map of current dew point
temperature values across the US on the UA Atmospheric Science
dept. website.
At the end
of the class period I asked you to be on the lookout, as you were
reading through the beginning of Chapter 1, for information about how
the earth's atmosphere might have changed over time.
The earth is about 4.5 billion years old. The earth's original
atmosphere is thought to have been composed mainly of hydrogen, helium,
and compounds like methane (CH4) and ammonia (NH3).
These gases
escaped into space. The next atmosphere is thought to have been
come from gaseous emissions from volcanoes and contained mostly water
vapor, carbon dioxide, and nitrogen. There was very little oxygen
in early in this second atmosphere. Oxygen was probably initially
produced by the photodissociation of water vapor and carbon dioxide (by
high energy UV light).
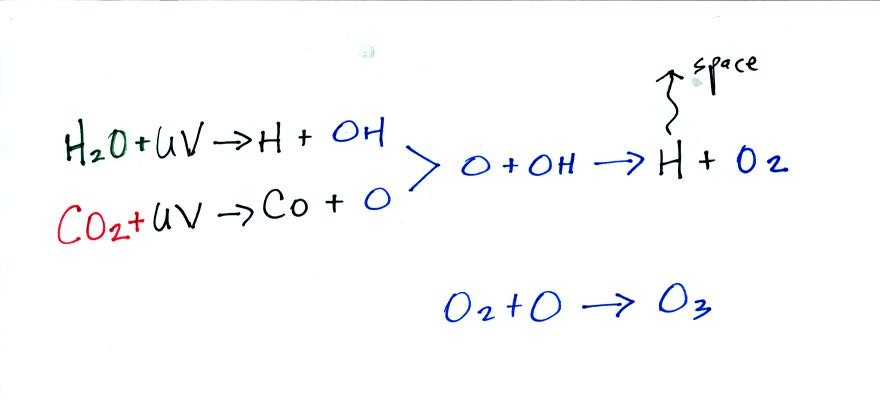
Once oxygen (O2) began to build up in the atmosphere, ozone
(O3) could
begin to form. Ozone would begin to absorb ultraviolet light and
life was able to move from the oceans onto land. Plants and
photosynthesis would become and is now the most main source of oxygen
in the atmosphere.