Friday Jan. 13, 2006
In the first few minutes of class I reviewed the information on dew
point temperature and evolution of the earth's atmosphere. This
material was not covered in class on Wednesday but was stuck on the end
of the Jan. 11 notes nonetheless.
Signup sheets for the experiments and book report were also circulated
through class. Names will be transferred to the online Report Signup Lists.
We'll
spend a couple of class periods covering some of the principal
atmospheric pollutants. We started today with sulfur
dioxide. You'll find sulfur dioxide discussed on pps 11-13 in the
packet of photocopied class notes.

Sulfur dioxide is produced by the combustion of sulfur containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless.
Sulfur dioxide concentrations lower than the National Ambient Air
Quality Standards listed above (established by the Environmental
Protection Agency) should not present a health risk. Note however
that levels of 1 ppm (part per million, one SO2 molecule
mixed in with
one million air molecules) does present a health risk to certain
people. SO2 concentration did exceed this value in
some of the
air pollution events listed in the next figure.

The Great London smog is still the deadliest air pollution event in
history. A stable air layer next to the ground can't mix with
cleaner air above.
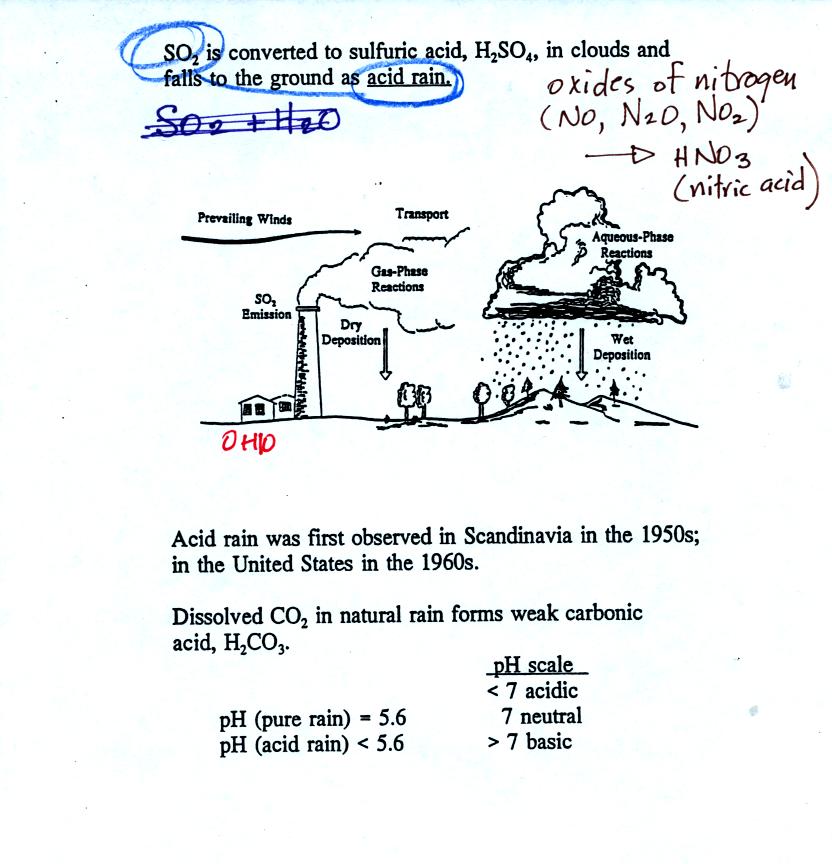
Acid rain often falls hundreds or thousands of miles away from the
source of the SO2. Coal fired factories and electric
power plants
in the Ohio River Valley could produce acid rain in New England and
Canada. Acid rain in Scandinavia could be the result of SO2
emissions in England and Belgium.

An acid rain demonstration was performed in the
last 15 minutes of class to give you a general idea of how acid rain is
produced. Carbon dioxide rather than SO2 was bubbled
through
Tucson tap water. The tap water is initially slightly basic (pH
> 7). Dissolved CO2 however turned the tap water
acidic.
The
following information was not covered in class.

Small drops of sulfuric acid that formed in the stratosphere following
the Mt. Pinatubo eruption reflected incoming sunlight. With less
sunlight arriving at the ground this lowered average temperatures at
the ground slightly for a period of a few years.
The acid
rain demonstration involved carbon dioxide. Carbon dioxide is an
important greenhouse gas. There is worldwide concern about
increasing atmospheric concentrations of CO2 and other
greenhouse
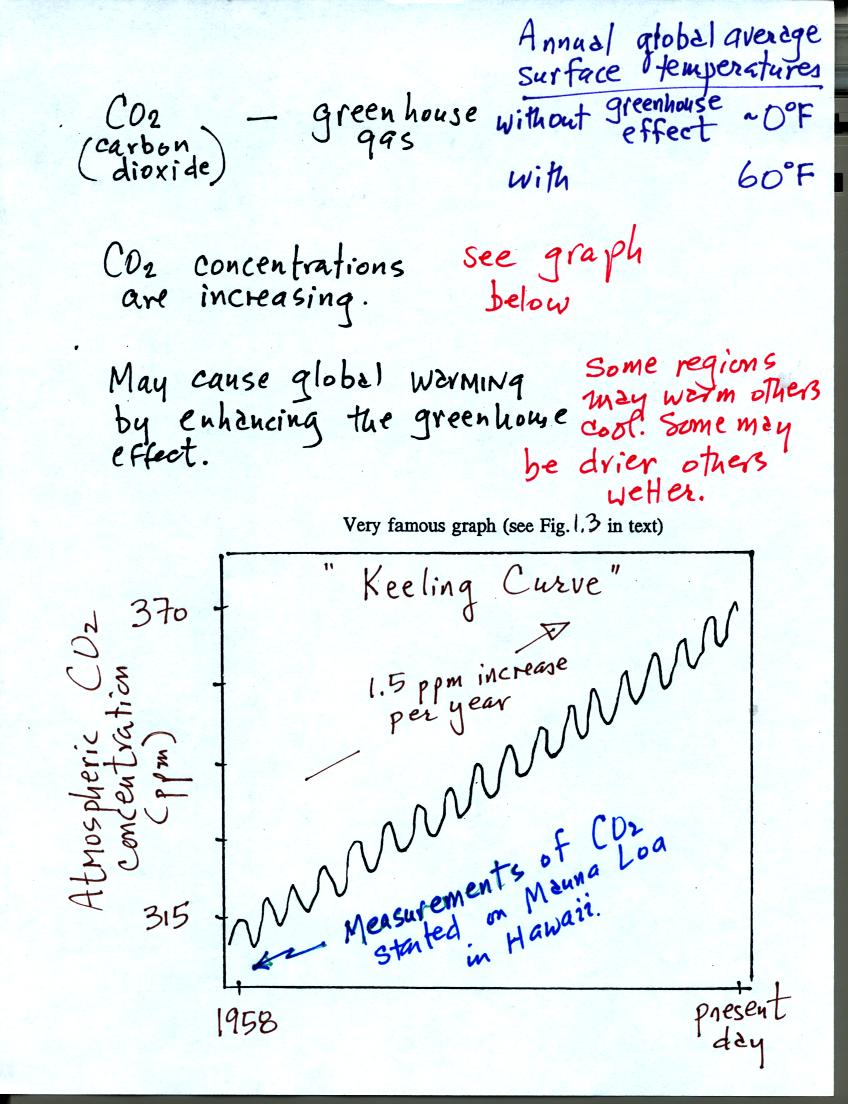
gases. The following figure is found on page 1 in the class notes.
We'll cover the greenhouse effect in detail in Chapter 2.
The natural greenhouse effect raises the overall average surface
temperature on the earth. The average annual global average would
be about 0o F without greenhouse gases in the
atmosphere. With
greenhouse gases the average is a much more pleasant 60o
F.
The concentration of CO2 (and other greenhouse gases) is
increasing. The Keeling curve shown above (and in Fig. 1.3 in the
text) clearly shows this. The concentration has increased from
about 315 ppm in 1958 when the measurements were started to about 370
ppm at present.
There is concern that increasing greenhouse gas concentrations may
strengthen or enhance the greenhouse effect and increase the global
average surface temperature. This could have a variety of
consequences that we will examine later.
We will first look at what is causing atmospheric CO2
concentrations to
increase. Before we do that we need to see how CO2 is
added to
and removed from the atmosphere.
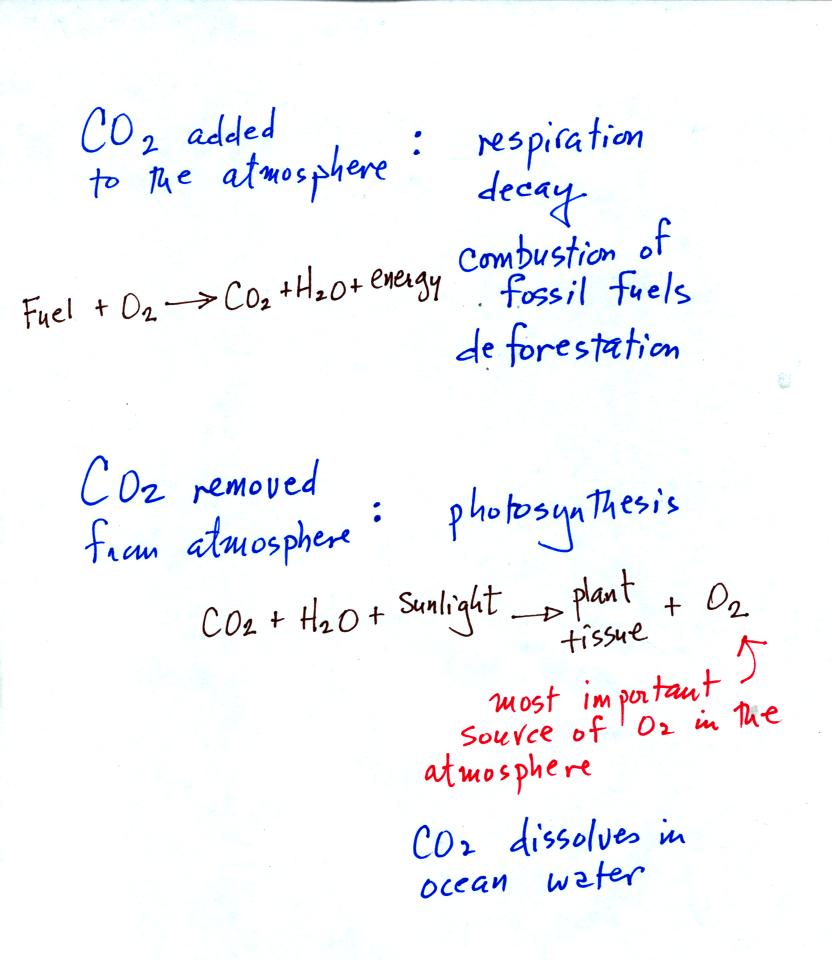
Natural processes such as respiration and decay add CO2 to
the
atmosphere. Volcanoes are an additional natural source.
Combustion and deforestation and human activities that add CO2
to the
air.
Photosynthesis removes CO2 from the air and is the main
source of
atmospheric oxygen. We saw how easily CO2 gas
dissolved in water
in the acid rain demonstration. CO2 is removed from
the
atmosphere when it dissolves in the oceans.
Knowing something about the sources and sinks of atmospheric CO2
we
can explain the wavy appearance in the Keeling curve. It
takes one year to complete one cycle.
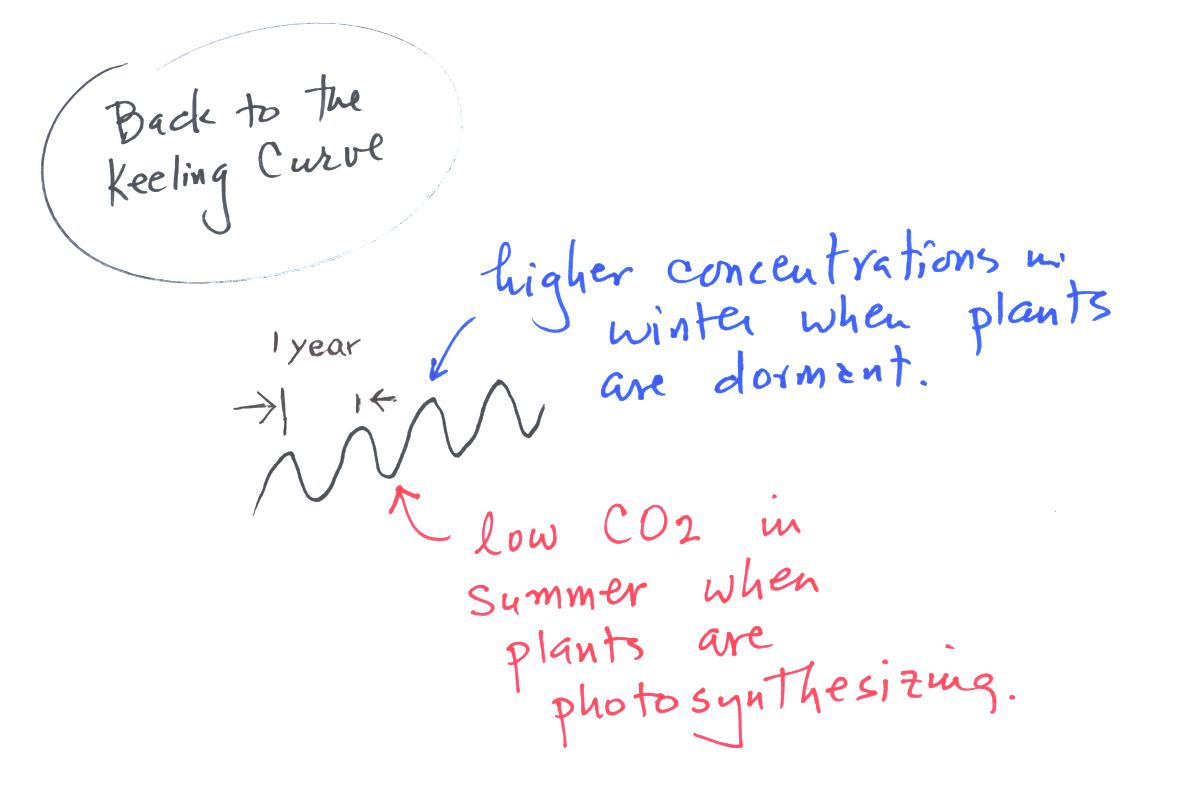
CO2 conentration peaks in the winter when plants are dormant
and are
not removing as much CO2 from the atmosphere during
photosynthesis. In the summer when plants are growing the
concentration of CO2 concentration decreases slighly.







