This somewhat confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized.
First, Point 1, the earth is thought to be between 4.5 and
4.6 billion years old.
The iron catastrophe was an important event (but wasn't
discussed in class). Circulation of liquid metal in the core of
the earth gives the earth a magnetic field. The magnetic field
deflects the solar wind around the earth. Remember the solar wind
may have swept away the earth's original atmosphere.
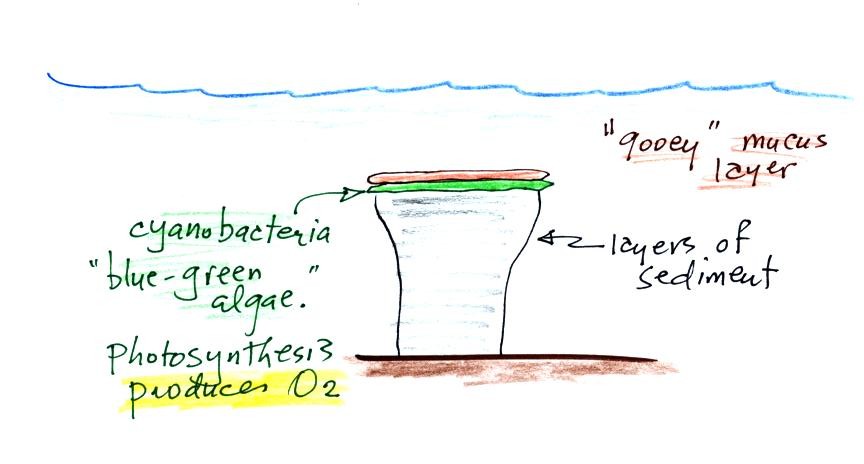
Stromatolites (Points 2 and 3) are column-shaped structures made
up of layers of sedimentary rock, that are created by microorganisms
living at the top of the stromatolite (note I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on the earth. Much older (3.5 to 3.8
B years old) stromatolites presumably produced by microbes but without
microbe fossils have also been found.

Living stromatolites are found in a
few locations today. The picture above is from Coral Bay Australia, located on the
western tip of the continent.
Once cyanobacteria began to produce
oxygen in ocean water, the oxygen reacted with dissolved iron (iron
ions in the figure below) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed.
Periodically the oxygen production would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
might have slowed the photosynthesus). During these times of low
dissolved oxygen concentrations, layers of jasper would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded iron formation. A couple of small polished pieces of
banded iron rock (actually "tiger iron") were passed around
class. In addition to the red and black layers, the tiger
iron contains yellow layers made of fibers of quartz.

Eventually the dissolved iron in
the ocean was used up (Point 4 in the timeline figure above).
Oxygen produced by cyanobacteria diffused from the ocean into the
atmosphere. Once in the air, the oxygen could react with iron in
sediments on the earth's surface. This produced red colored
sedimentary rock. None of these socalled red beds are older than
about 2 B years old. Thus it appears that a real buildup up
oxygen began around 2 B years ago. Oxygen concentrations reached levels
that are about the same as today around 500 to 600 years ago (Point 5
in the figure).
We listed
the 5 most abundant gases in the atmosphere in class on Monday.
Several more important trace gases were added to the
list in
class today. Trace gases are gases found in low
concentrations. Low concentrations doesn't mean they aren't
important, however.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing gas),
chlorofluorocarbons, and ozone are all greenhouse gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic more next week and learn more about how the
greenhouse effect actually works later in the course.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants. We'll cover carbon
monoxide on Friday and talk about sulfur dioxide and ozone next week.
Be careful with ozone:
(i) Ozone in the
stratosphere (a layer of the atmosphere between 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
We have
been discussing the composition of air. Air is mostly composed on
invisible gases. This past summer I thought it might be
interesting to bring in several examples of gases that you can actually
see (the gases are colored, not clear; you can't of course see the
individual gas atoms or molecules). Once I started to do some
research I found that many of these gases are very poisonous. In
some cases a sample large enough for you to be able to see would be a
potentially fatal dose if it were to be released accidentally into the
classroom. You're going to have to settle for pictures of chlorine (a gas with a
yellow-green color), and bromine
(a liquid that evaporates, the resulting gas has a very vivid reddish
color). The caution on the www.webelements.com website: "Bromine
is a serious health hazard and maximum safety precautions should be
taken when handling it" worried me a little bit. I will bring in
some iodine (a solid
that sublimates producing a gas with a faint pink color) later in the
semester (it's poisonous but not nearly as scary as some of these other
gases).
We did however make some nitrogen dioxide,
a toxic pollutant. We did this by putting an ordinary copper
penny (Cu(s) in the equation below) into a large 4 liter glass flask
that contained a small amount of concentrated nitric acid ( HNO3(aq) ).

Air
Pollution is a serious health hazard in the US and around the
world. The following statistics were shown briefly at the end of
class. A few additional details were added after
class. Click here
to download a copy of this handout (which was not distributed in class).
Keep in mind that many of these numbers are difficult to measure
and some may contain a great deal of uncertainty. The row that is
highlighted, toxic agents, contains estimates of deaths caused by
indoor and outdoor air pollution, water pollution, and exposure to
materials such as asbestos and lead both in the home and at the work
place. It is estimated that 60% of the deaths are due to exposure
to particulate matter, something that we will examine in a little more
detail next week.

Air pollution is a serious hazard
worldwide. Interestingly indoor air pollution is, in many places,
a more serious threat than outdoor air pollution.
The Blacksmith
Institute has listed the Top 10 polluted places in the world in a
2007 report. The report has received a lot of worldwide
attention. If you go to this
address, you can view the report online or download and print a
copy of the report. Just in case you are interested.
That's about as far as we got in class on Monday, I won't add any new
material to today's online notes that wasn't covered in class. We'll
start with the section on Carbon Monoxide (pps 7-10) in the photocopied
ClassNotes in class on Friday.








