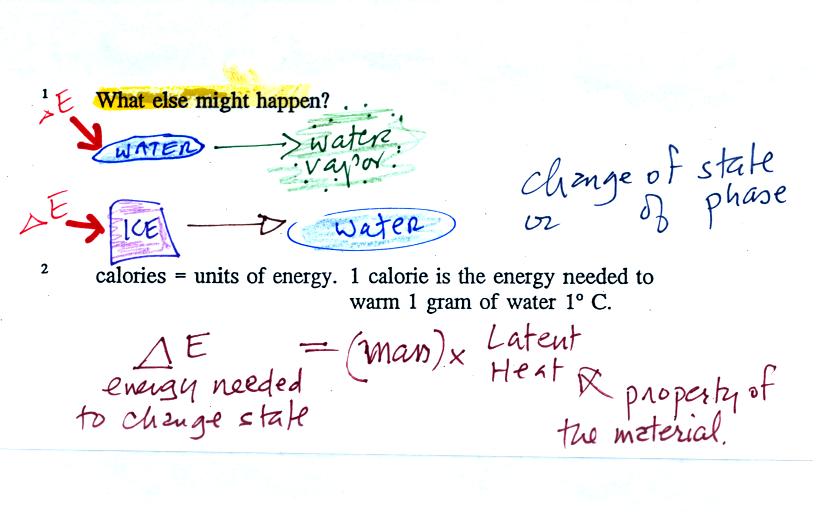
The temperature change will
first depend on
how much energy was added. This is a direct proportionality, so
delta E is in the numerator of the
equation.
When you add equal amounts of energy to large and small pans of water, the small pan will heat up more quickly. The temperature change, delta T, will depend on the mass. A small mass will mean a large delta T, so mass should go in the denominator of the equation.
Different materials react differently when energy is added to them. A material with a large specific heat will warm more slowly than a material with a small specific heat. Specific heat behaves in the same kind of way as mass. Specific heat is sometimes called "thermal mass" or "thermal capacity."
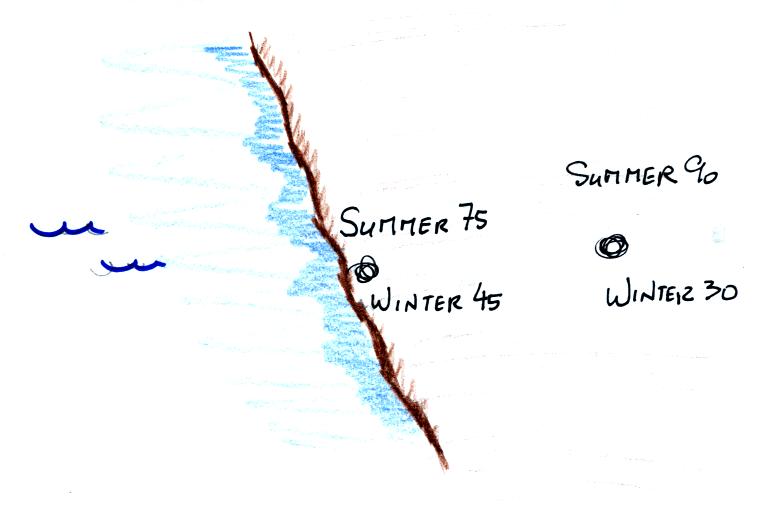
Here's an important example that will show the effect of specific heat (middle of p. 45)

When you add equal amounts of energy to large and small pans of water, the small pan will heat up more quickly. The temperature change, delta T, will depend on the mass. A small mass will mean a large delta T, so mass should go in the denominator of the equation.
Different materials react differently when energy is added to them. A material with a large specific heat will warm more slowly than a material with a small specific heat. Specific heat behaves in the same kind of way as mass. Specific heat is sometimes called "thermal mass" or "thermal capacity."
Here's an important example that will show the effect of specific heat (middle of p. 45)