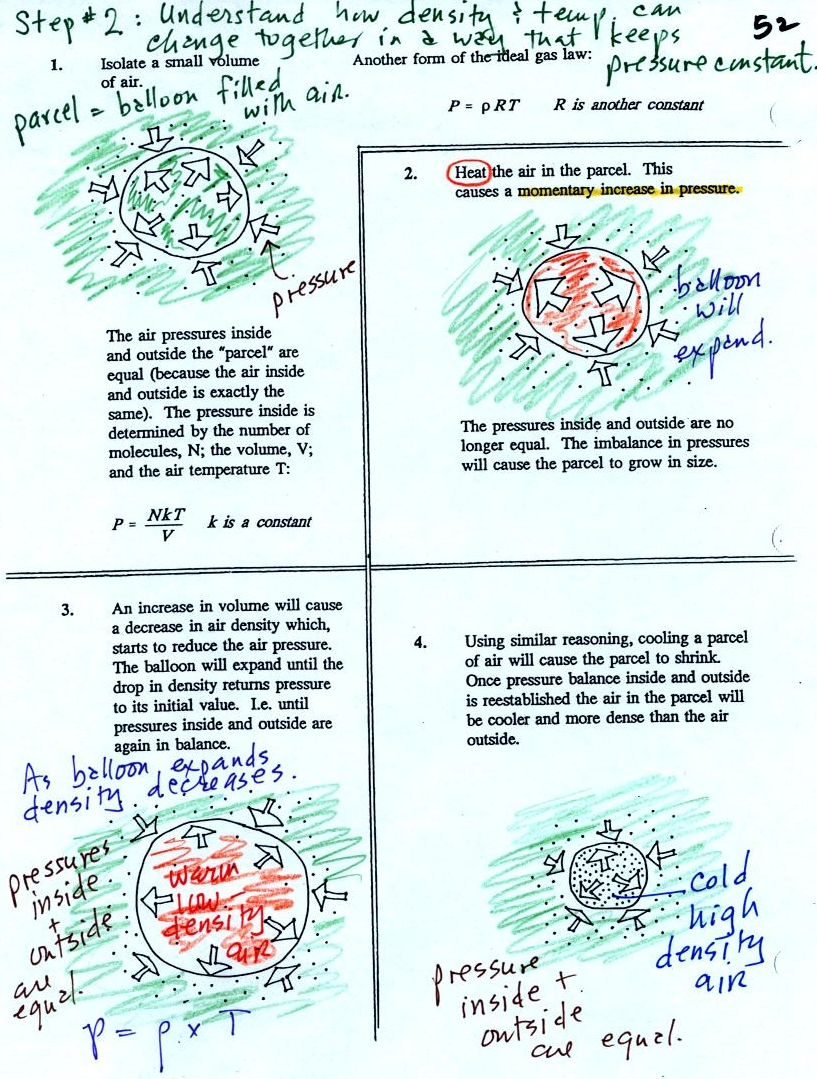
Read through the explanation on p.
52 in the photocopied
Classnotes.
These two associations:
(i)
warm air = low
density air
(ii) cold air = high density air
are important and will come up a
lot during the remainder of the
semester.
Click here if you
would like a little
more detailed, more step-by-step,
explanation of Charles Law. Otherwise proceed on to the Charles'
Law demonstration that we did in class.
Charles
Law can be demonstrated by dipping a balloon in
liquid
nitrogen. You'll find an explanation on the top of p. 54 in the
photocopied ClassNotes.
The balloon had shrunk down to practically no volume when
pulled from the liquid nitrogen. It was filled with cold high
density air. As
the balloon warmed the balloon expanded and the density of the air
inside
the balloon decreased. The volume and temperature kept changing
in a way that kept pressure constant.
Here's a summary (not shown in
class)
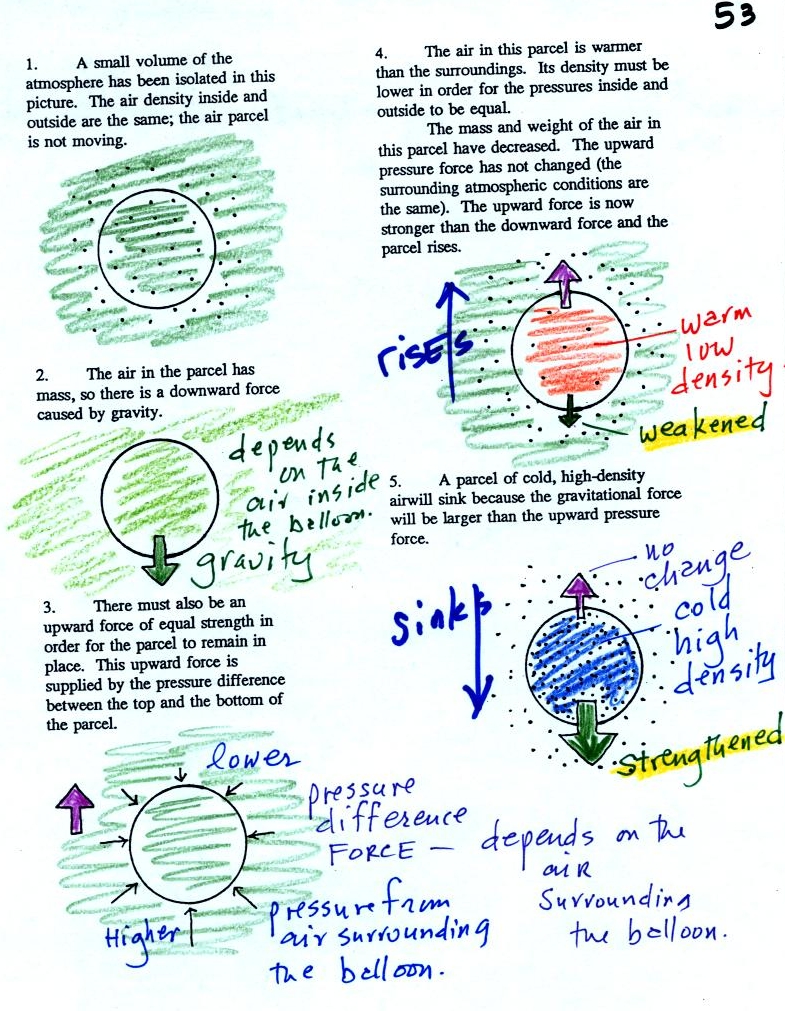
Now
finally on to step #3. Found on p. 53 in the photocopied
ClassNotes.
Basically what it comes down to is this - there are two forces
acting on a parcel (balloon) of air:
Gravity pulls downward. The strength of the gravity force depends
on the mass of the air inside
the balloon.
There is an upward pointing pressure difference force. This is
caused by the air surrounding the balloon.
When the air inside a parcel is exactly the same as the air outside,
the two forces have equal strengths and cancel out. The parcel is
neturally bouyant and doesn't rise or sink.
If you replace the air inside the balloon with warm low density air, it
won't weigh as much. The gravity force is weaker, the upward
pressure difference force wins and the balloon will rise.
Conversely if the air inside is cold high density air, it weighs
more. Gravity is stronger than the upward pressure difference
force and the balloon sinks.
To
demonstrate free convection we modified the Charles Law
demonstration. We used
balloons filled with hydrogen instead of air (see bottom of p. 54 in
the photocopied Class
Notes). Hydrogen is less dense than air even when the
hydrogen has the same temperature as the surrounding air. A
hhydrogen-filled balloon doesn't need to warmed up in order to rise.
We dunked the helium-filled balloon
in some liquid nitrogen to cool
it
and to cause the density of the helium to increase. When
removed
from the liquid nitrogen the balloon didn't rise, the gas inside was
denser than the surrounding air (the purple and blue balloons in the
figure above). As the balloon warms and expands
its density decreases. The balloon at some point has the same
density as the air around it (green above) and is neutrally
bouyant. Eventually the balloon becomes less dense that the
surrounding air (yellow) and floats up to the ceiling.
Here's an example of where something like this happens in the
atmosphere.
At (1) sunlight reaching the ground is absorbed and warms the
ground. This in turns warms air in contact with the ground
(2). Once this air becomes warm and its density is low enough,
small blobs of air separate from the air layer at the ground and begin
to rise (3). This is called free convection; many of our summer
thunderstorms start this way.