Friday Feb. 20, 2009
you can download today's notes in a more printer friendly format
by clicking here
A tough crowd to please this afternoon, music-wise. I think we
finally settled on Buckwheat
Zydeco.
Quiz #1 is graded and was returned in class. The class average
was pretty high: 79%. Check to be sure your quiz was graded
correctly and that the points were added up correctly. Note you
can only earn up to 10 pts on the extra credit questions (I usually put
that in parentheses but forgot this time).
During the next few weeks we will be concerned with energy,
temperature, heat, energy transport, and
energy
balance between the earth, atmosphere, and space.
It is easy to
lose sight of the main concepts because there are so many
details. The following (found on pps 43&44 in the photocopied
Class Notes) is meant to introduce some of what we will be covering in
class.

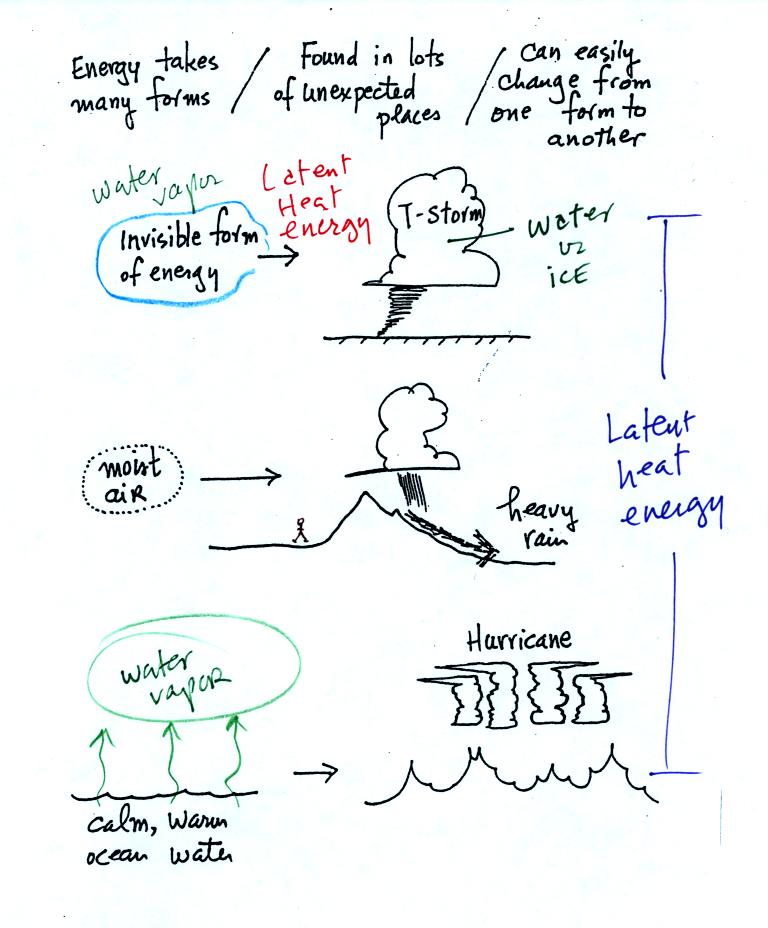
Latent heat energy is perhaps the most underappreciated and most
confusing type of energy. The word latent refers to energy that is
hidden in water and water vapor. The hidden energy emerges when
water vapor condenses or water freezes.
Radiant energy is a very important form of energy that was for some
reason left off the original list. Sunlight is an example of
radiant energy that we can see and feel (you feel warm when you stand
in sunlight). There are many types of radiant energy
that are invisible.
It is hard to visualize or appreciate the amount of energy released
into the
atmosphere during condensation. You can imagine the work that you
would do carrying a gallon of water
(8 pounds) from Tucson to the top of Mt. Lemmon. To
accomplish
the same thing Mother Nature must first evaporate the water and (if my
calculations are correct) that requires about 100 times the energy that
you would use to carry the 8 pounds of water to the summit of Mt.
Lemmon. And Mother Nature transports a lot more than just a
single gallon.
Four energy transport
processes are listed below.
By far the
most important process is electromagnetic radiation (light is a common
form of electromagnetic radiation). This is the
only process that can transport energy through empty space.
Electromagnetic radiation travels both to the earth (from the sun) and
away from the earth into space. Electromagnetic radiation is also
responsible for about 80% of the
energy transported between the ground and atmosphere.
You might be
surprised to learn that latent heat is the second most important
transport process.
Rising parcels of warm air and sinking parcels of cold air are
examples of free convection. Because of convection you feel
colder or
a cold windy day than on a cold calm day.
Ocean currents are also an example of convection. Ocean currents
transport energy from the warm tropics to colder polar regions.
Note that convection is
a 3rd way of causing rising air motions in the atmosphere (convergence
into centers of low pressure, and fronts were the other two
ways).
Conduction is the least important energy transport at least in the
atmosphere. Air is such a poor conductor of energy that it is
generally considered to be an insulator.
Water vapor is a particularly important form of invisible
energy.
When water vapor condenses to produce the water droplets (or ice
crystals) in a
cloud, an enormous amount of latent heat energy is released into the
atmosphere.
It is hard to visualize or appreciate the amount of energy released
into the
atmosphere during condensation. You can imagine the work that you
would do carrying a gallon of water
(8 pounds) from Tucson to the top of Mt. Lemmon. To
accomplish
the same thing Mother Nature must first evaporate the water and (if my
calculations are correct) that requires about 100 times the energy that
you would use to carry the 8 pounds of water to the summit of Mt.
Lemmon. And Mother Nature transports a lot more than just a
single gallon.
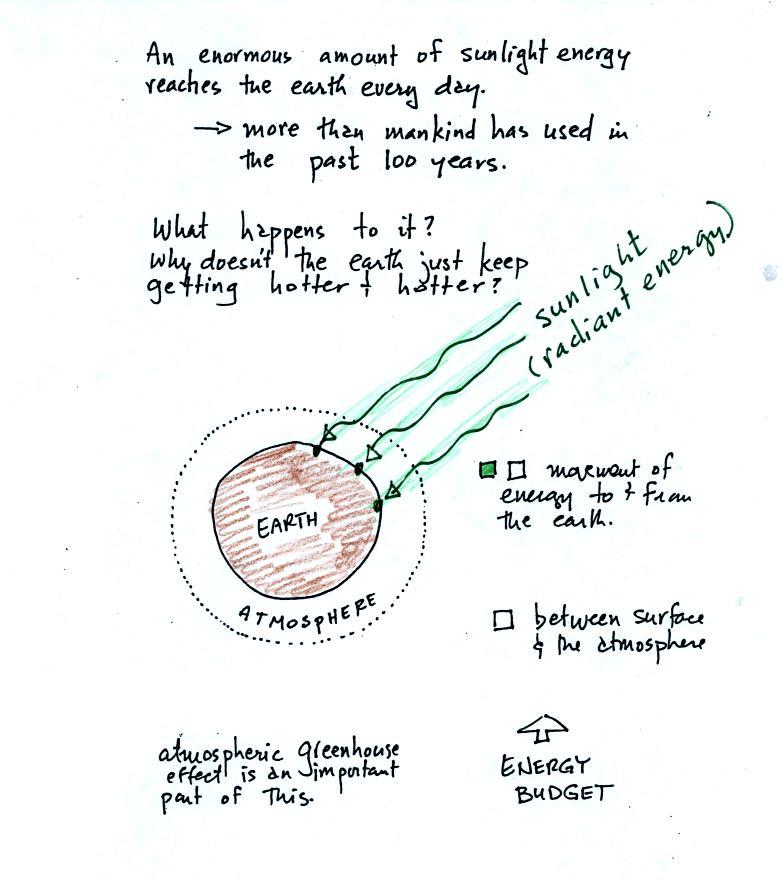
The next picture shows energy being transported from the sun to
the earth in the form of electromagnetic radiation.
We are aware of this energy because we can see it (sunlight
also contains invisible forms of light) and feel it. With all of
this energy arriving at and
being
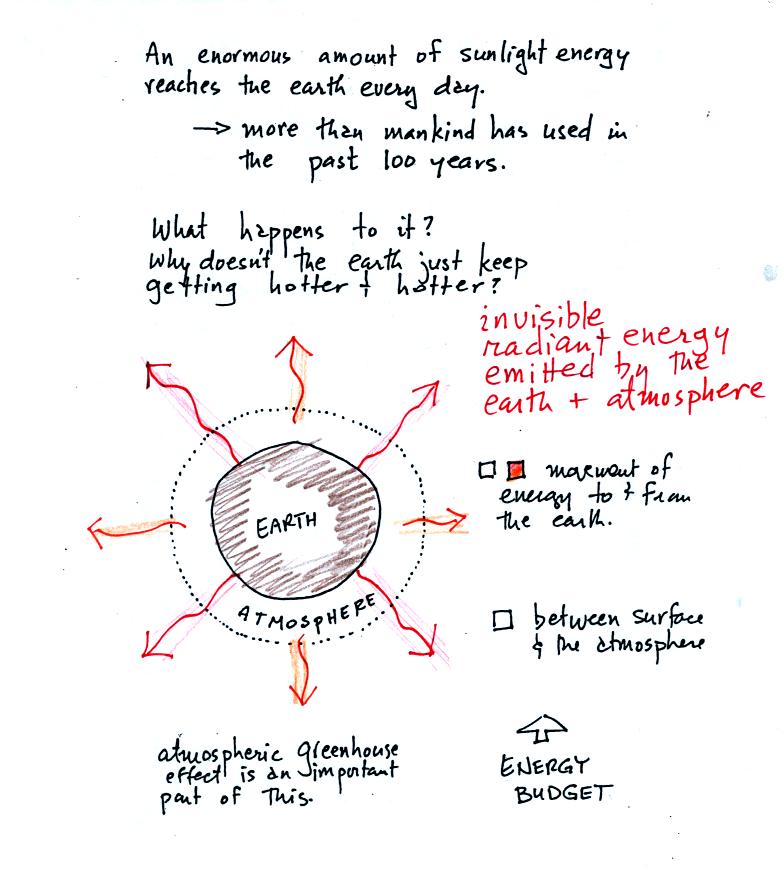
absorbed by the earth, what keeps the earth from getting hotter and
hotter? The answer is that the earth also sends energy back into
space (the orange and pink arrows in the figure below)
This infrared light is an
invisible form of energy (it is weak enough that we
don't usually feel it either). A balance
between incoming and outgoing energy is achieved and the earth's annual
average temperature remains constant.
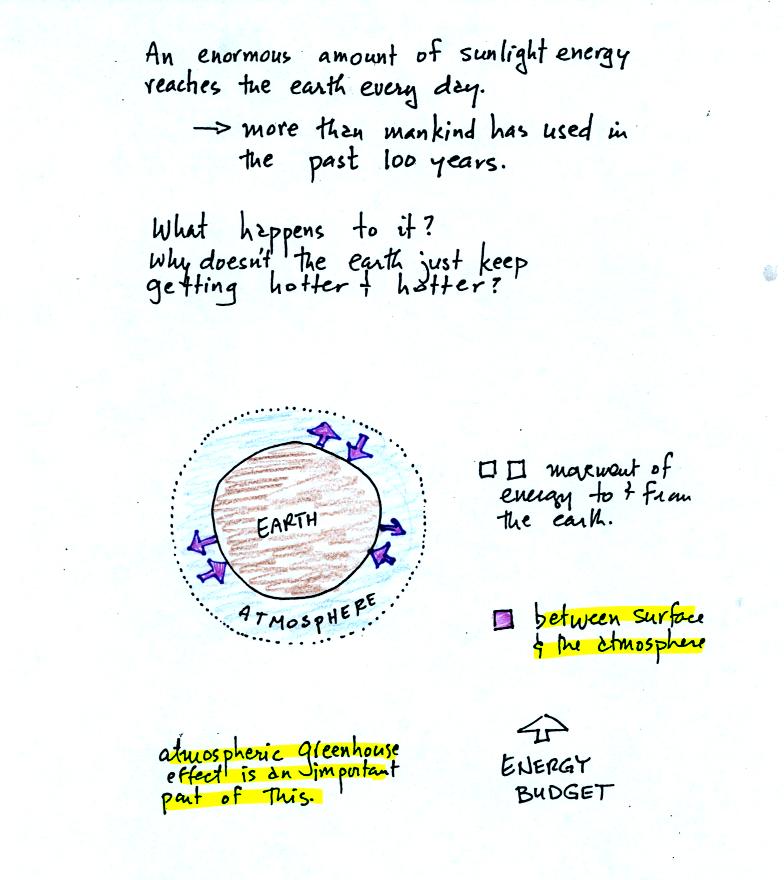
We will also look closely at energy transport between the earth's
surface and the atmosphere. This is where latent heat energy transport
and convection and conduction operate (they can't outside
the atmosphere into outer space).
That is also where the atmospheric
greenhouse operates. That will be a important goal -
to
better understand how the atmospheric greenhouse effect works.
Remember that without the greenhouse effect, the global annual
average surface temperature on the earth would be about 0o F
rather
than 60o F.
When you
add energy to an object, the object will usually
warm
up (conversely when you take energy from an object the object will
cool). It is relatively easy to come up with an equation that
allows
you to figure out what the temperature change will be.
The temperature change will
first depend on
how much energy was added. This is a direct proportionality, so
delta E is in the numerator of the
equation.
When you add equal amounts of energy to large and small pans
of water, the small pan will heat up more
quickly. The temperature change, delta T, will depend on the
mass. A small mass will mean a large delta T, so mass should go
in the denominator of the equation.
Different materials
react differently when energy is added to them. A material with a
large specific heat will warm more slowly than a material with a small
specific heat. Specific heat has the same kind of effect on delta
T as
mass. Specific heat is sometimes called "thermal mass" or
"thermal capacity."
Here's an important example that will show the effect of specific
heat (middle of p. 45)
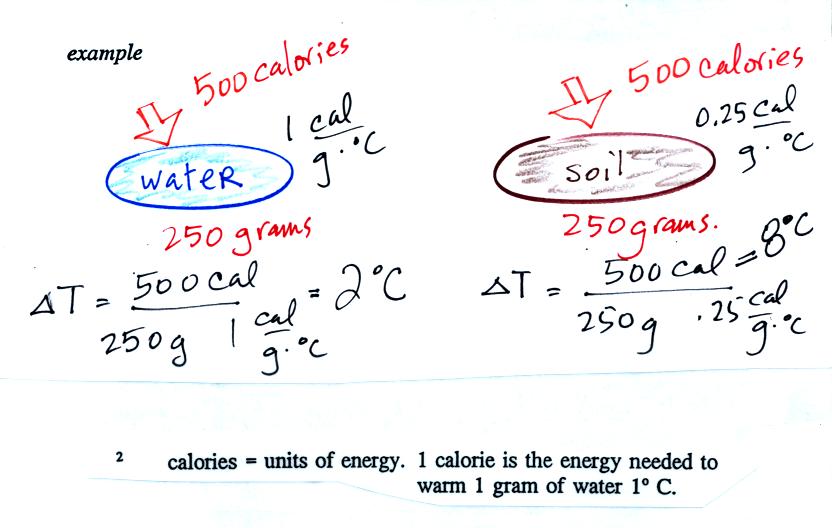
Equal
amounts of energy (500 calories, note that calories are units of
energy) are added to
equal masses (250 grams) of water and soil. We use water and soil
in the
example because most of the earth's surface is either ocean or land.
Water has a higher specific heat than soil, it only warms up 2o
C.
The soil has a lower specific heat and warms up 8o C, 4
times more
than the water.
These different rates of warming of water and soil have
important effects on regional climate. (the following figure was not shown in
class)
Oceans moderate the climate.
Cities near a large body
of water won't warm as much in the summer and won't cool as much during
the winter compared to a city that is surrounded by land.
The city above on the
coast has a 30o F annual range of temperature (range is the
difference between the summer and winter temperatures). The
city further
inland (assumed to be at the same latitude and altitude) has an annual
range of 60o F. Note that both cities have the same 60o
F annual
mean temperature.
Adding
energy to an object will usually cause it to warm. But there
is another possibility (bottom p. 45), the object could change
phase (change
from solid to liquid or
gas). Adding energy to ice might cause
the
ice to melt. Adding energy to water could cause it to evaporate.
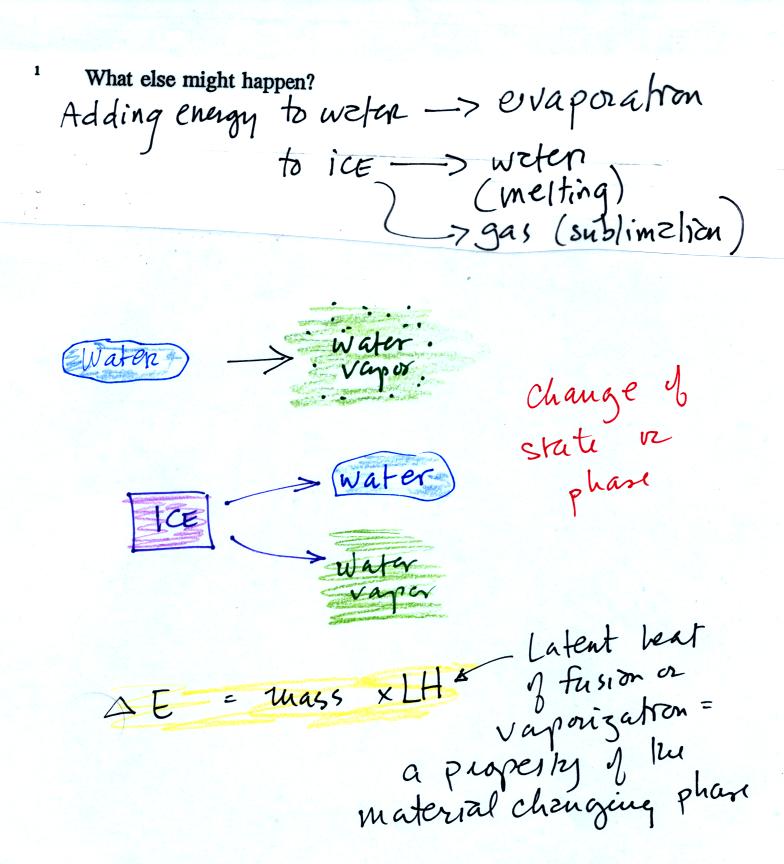
The equation at the bottom of the figure above allows you to
calculate how much energy is required to melt ice or evaporate water or
sublimate dry ice. You multiply the mass by the latent heat, a
variable that depends on the particular material that is changing
phase.
Next we learned a little bit about the Piccard family.
Auguste Piccard (together with Paul Kipfer, see p. 32 in the
photocopied ClassNotes) was the lead member of a two-man team that made
the first trip into the stratosphere in a balloon. They did that
on May 27, 1931. A short video was shown in class
describing their trip.
Jacques Piccard (Auguste's son) was part of a two-man team that
traveled to the deepest point in the ocean (35,800 feet) in a
bathyscaph. You'll see a short segment from a early test
of the bathyscaph where Auguste and Jacques descend to 10,000 feet.
Finally Bertrand Piccard (Jacques' son, Auguste's grandson) was part of
the two man team that first circled the globe nonstop in a
balloon. That occurred fairly recently, March 20, 1999, I
believe. I also plan to show you some of that trip also.