Monday Feb. 23, 2009
click here to download today's notes in
a more printer friendly format.
Music today was The
Ballad of Cable Hogue from Calexico. Calexico is a local
group and appear fairly frequently at the Rialto theater in
Tucson. They are just finishing up a European tour and will be
appearing at Carnegie Hall on Mar. 11. On the DVD shown in class
they were appearing together with Mariachi Luz de Luna (also from
Tucson) and Francoiz Breut at the Barbican Theater in London.
The Experiment #2 reports are due Monday
next week. Try to return your materials this week so that you can
pick up the Supplementary Information sheet. The Experiment #3 materials should be distributed
Wednesday or Friday next week.
Tomorow is Mardi Gras. Mardi Gras is the inspiration for
this week's picture
which can be viewed by clicking here.
Now back
to business. Here's a little review of some of what we covered
last
Friday.
If you add energy to or remove energy from an object, the
object
will usually change temperature. You can calculate the
temperature change if you know the object's mass and its specific
heat.
We will be using the equation in a slightly different way in class
today. We will measure the temperature change and use that to
determine the amount of energy lost by an object.
Another thing we learned was that water has relatively high specific
heat (4 or 5 times higher than soil for example). This has some
important consequences.
A city on a coast (especially the west coast) will have a
more
moderate climate than a city located inland (everything else being the
same). It won't get as hot in the summer and won't get as cold in
the winter. The annual range of temperature will be
smaller. Water's high specific heat means it is hard to heat the
water in the summer and hard to cool the water in the winter.
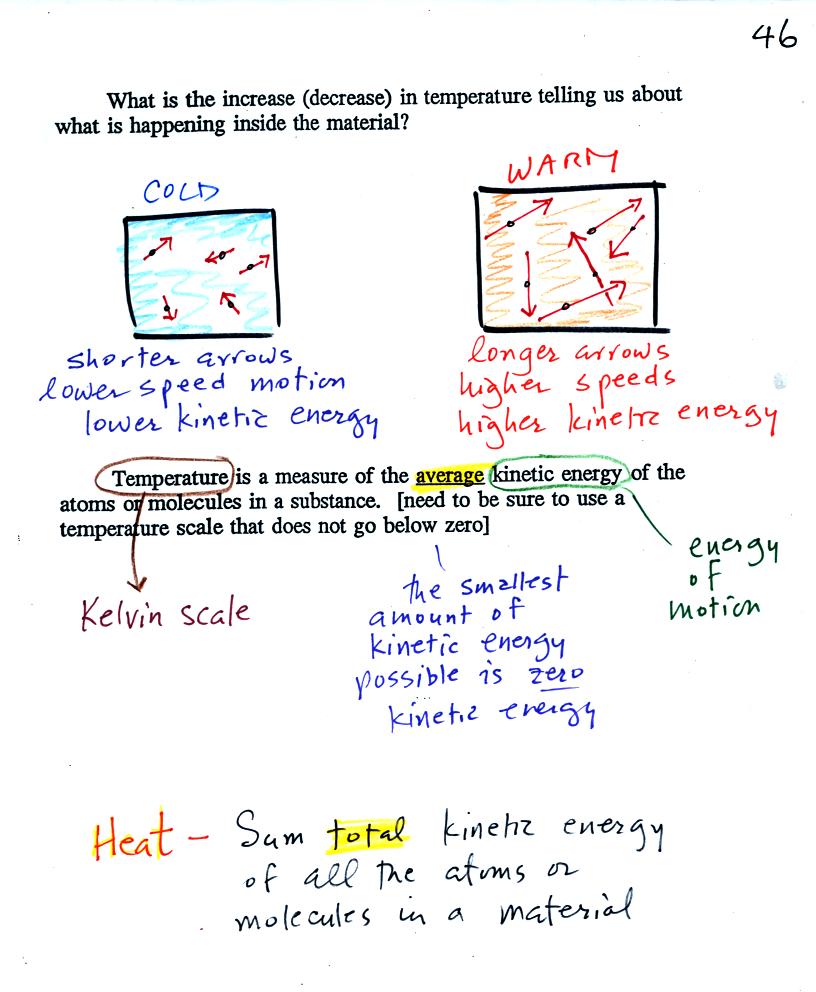
When you
add energy to an object and the object warms, what exactly is
happening inside the object?
The figure above is on p. 46 in the
photocopied Class
Notes. Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material. The atoms or molecules in a cold
material will be moving more slowly than the atoms or molecules in a
warmer object.
You need to be careful what temperature scale you use when
using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
The next figure might make the distinction between temperature (average
kinetic energy) and heat (total kinetic energy) clearer.
A cup of water and a pool of water
both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
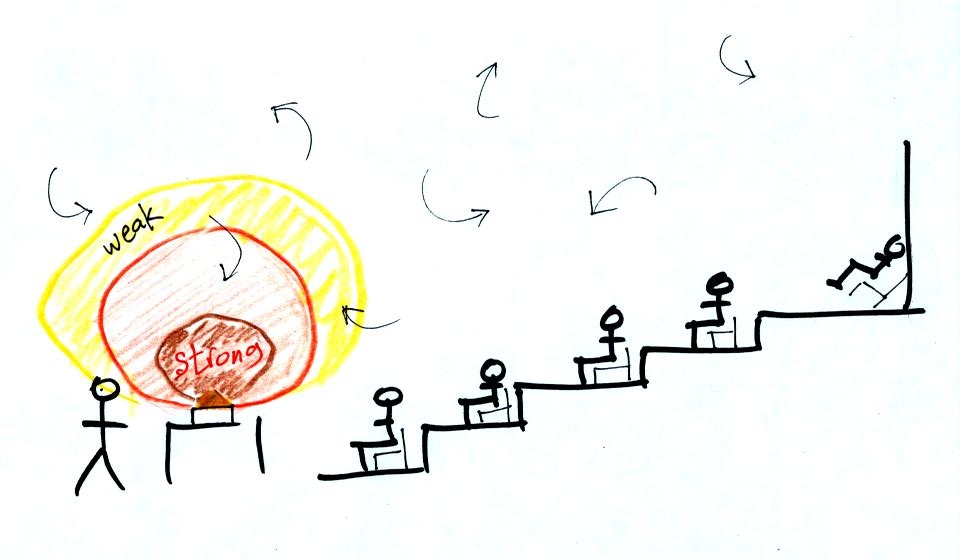
In the same way the two groups of people shown have the same
average
amount
of money per person. The $100 held by the larger group at the
left is
greater than the $20 total possessed by the smaller group of people on
the right.
Speaking
of temperature scales.
You should remember the
temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and Kelvin scales. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth.
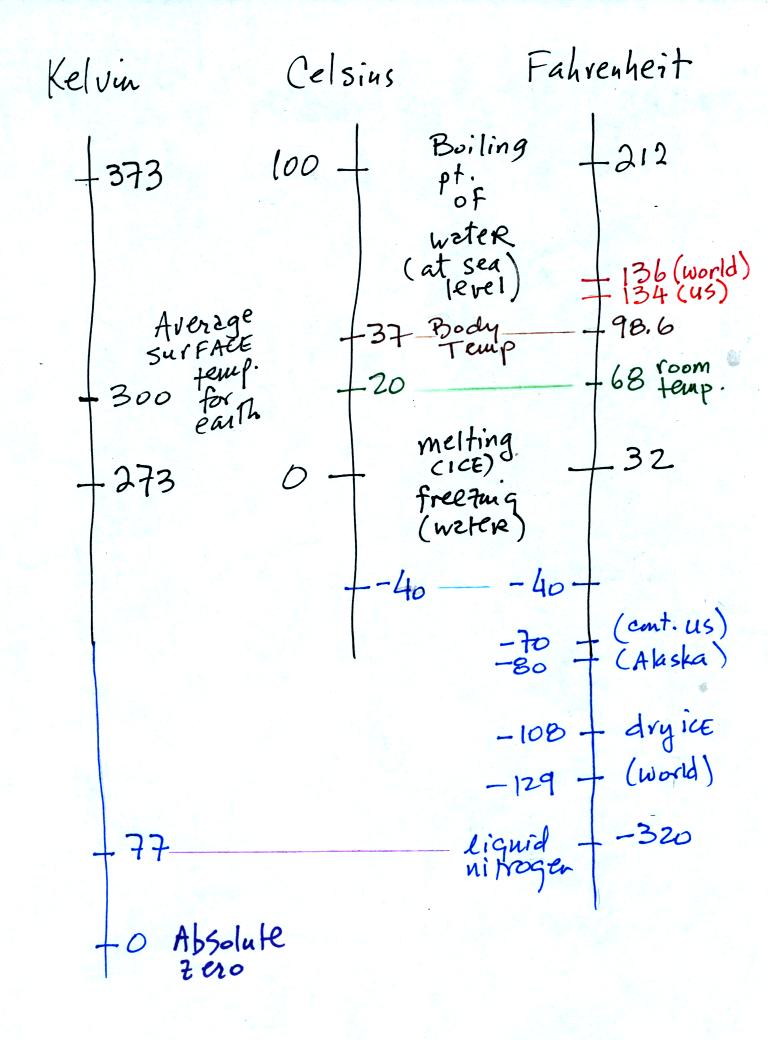
You certainly don't need to try to
remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean. Liquid
nitrogen is cold but it is still quite a bit warmer than absolute zero.
At this
point a student from the class was brave enough to volunteer to do an
experiment.
The object of the experiment was to
measure the latent heat of
vaporization of liquid nitrogen. That just means measuring the
amount of energy needed to evaporate a gram of liquid nitrogen.
The students that are doing Experiment #2 are measuring the latent heat
of fusion of ice, the energy needed to melt one gram of ice.
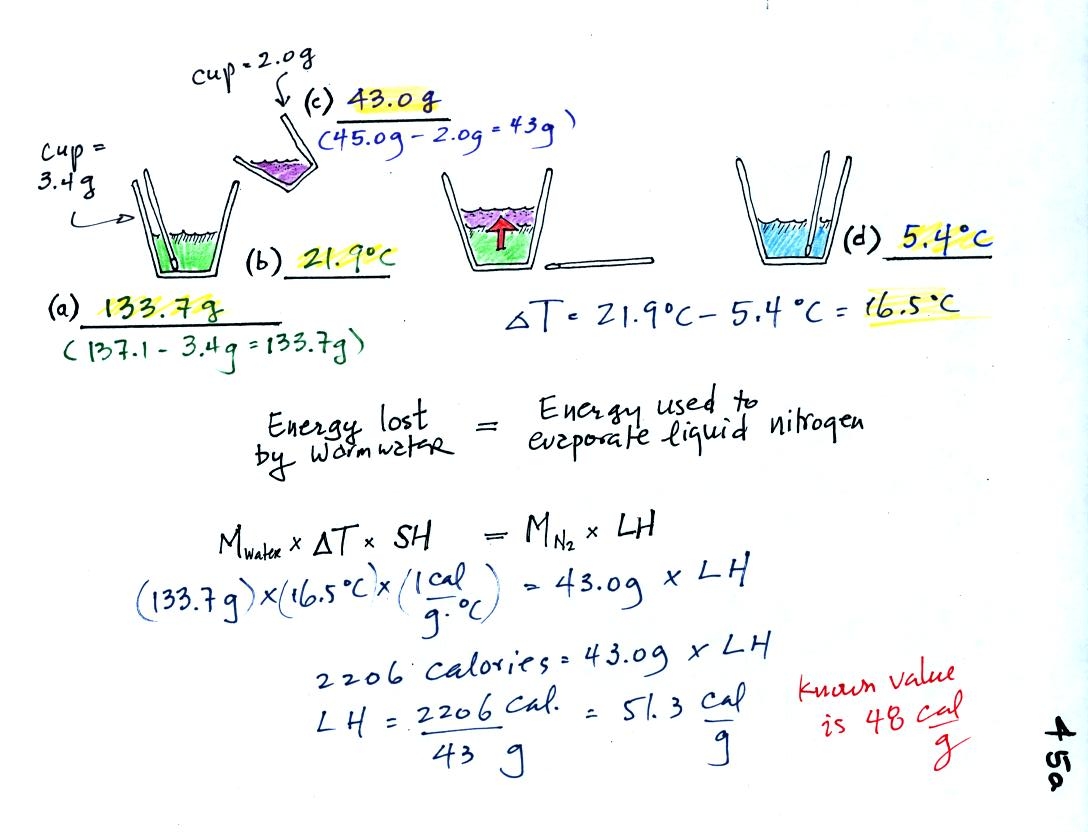
You'll
find the following figure on p. 45a in the photocopied
Classnotes.
(a)
Some room temperature water poured into a styrofoam cup weighed
137.1
g. The cup itself weighed 3.4 g, so we have 133.7 g of water.
(b)
The water's temperature was 21.7 C.
(c)
43.0 g of liquid nitrogen was poured into the cup of water.
It takes energy to turn liquid nitrogen into nitrogen gas.
The needed energy came from the water. This flow of energy is
shown in the middle figure above. We assumed that because the
experiment is performed in a styrofoam cup that there is no energy
flowing between the water in the cup and the surounding air.
(d)
After the liquid nitrogen had evaporated we remeasured the water's
temperature. It had dropped to 5.4 C. That is a
temperature drop of 16.5 C.
Because we knew how
much water we started with, its temperature drop, and water's specific
heat we can calculate how much
energy was taken from the water. That is the 2206 calorie
figure above. This was used to evaporate 43 grams of liquid
nitrogen. So we divided 2206 calories by 43 grams to get 51.3
calories needed per gram. That is our
measured value of the latent heat of vaporization of nitrogen. A
trustworthy student in the class informed us that
the known value is 48 cal/g, so our measurement
was pretty close.
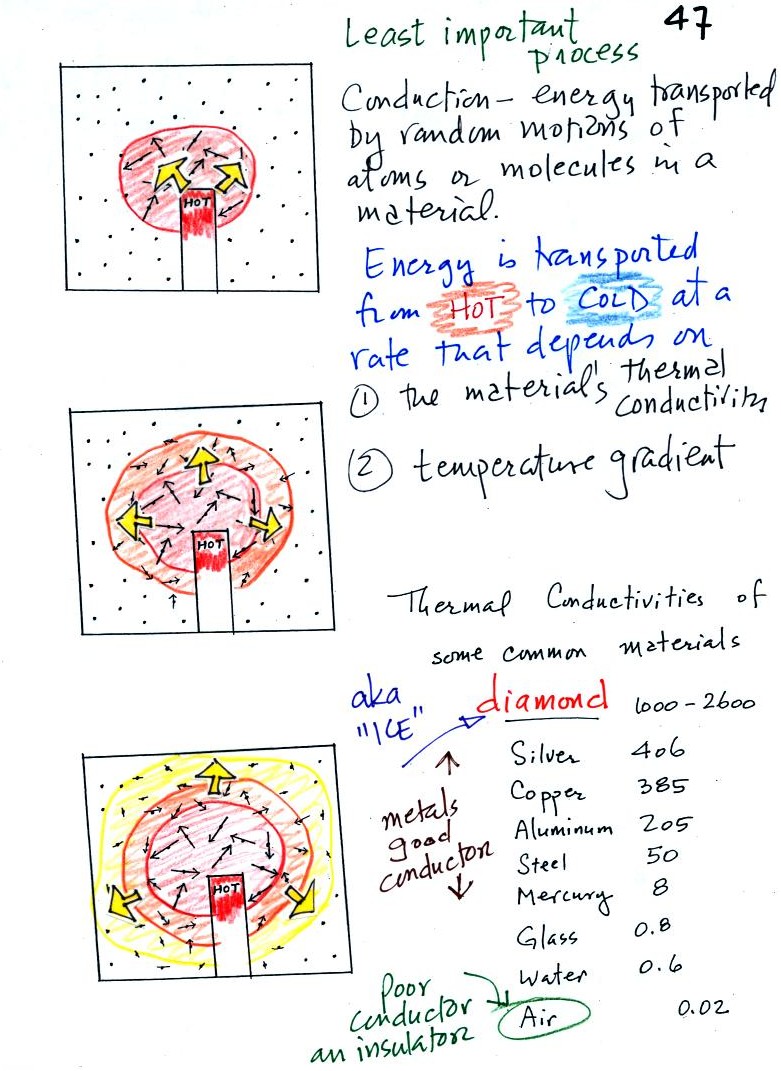
Conduction
is the first of four energy transport processes
that we
will cover. The figure below illustrates this process. A
hot object is stuck in the middle of some air.
In the top picture some of the
atoms or molecules near the
hot object have collided with the object and picked up energy from the
object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red).
In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object.
In
the third picture molecules further out have now (the yellow ones)
gained
some energy. The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
Conduction transports energy from hot to cold. The rate of
energy transport depends first on the material (air in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (sauce pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on
temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
Transport of energy by conduction is similar to the
transport of a strong smell throughout a classroom by diffusion.
Small eddies of wind in the classroom blow in random directions and
move smells throughout the room.. For our demonstration we used
curry powder.
The curry powder was actually
placed on a hot plate.
With time the smell should have spread throughout the room.
By the end of class some students in the back of the room said
they could detect just the faintest hint of the curry smell.