Quiz #2 Study Guide
Energy,
temperature and heat (15 pts). Kinetic energy -
energy of motion. Temperature (which scale?) provides a
measure of the average kinetic energy of the atoms or molecules in
a substance. Heat energy is the total kinetic energy of all
the atoms or molecules in a material. Energy units:
calories. What is the relationship between energy added to
(or removed from) an object, ΔE,
and the temperature change, ΔT,
that results? Specific heat (aka thermal mass or thermal
capacity). Water has a relatively high specific heat (4 or 5 times
higher than soil). A city on a coastline will have a more
moderate climate (what does that mean?) than a city located
further inland. Other than a change in temperature what else
can happen when energy is added to or removed from a material?
Temperature scales (15 pts).
Fahrenheit, Celsius, and Kelvin (absolute) scales.
You should know the temperatures of the boiling point of water at
sea level and the melting point of ice/freezing point of water on
the F and C scales. The global average surface temperature of the
earth is about what temperature on the Kelvin scale?
Energy transport (15 to
20 pts).
(1) Conduction. Energy is transported from hot to
cold by random atomic or molecular motions at a rate that depends
on the material (thermal conductivity) and the temperature
gradient. Examples of good and poor conductors. An object with
high thermal conductivity will often feel cold to the touch
because it rapidly conducts energy away from your body (our
perception of temperature is not a good measurement of
temperature).
(2) Convection. Energy transport by organized motion of atoms or
molecules (works in gases and liquids but not solids). Free
(rising and sinking air) and forced convection. Free convection is
a third way of causing rising air motions in the atmosphere.
Wind chill temperature.
Energy
transport (20 pts).
(3) Latent heat energy
transport. 2nd most important energy transport
process. Six phase change names. For each phase change you
should know whether energy is added to a material (absorbed from
or taken from the surroundings) or taken from the material
(released into the surroundings).
Sample Questions
Quiz #1: 5, 12, EC3 Final
Exam: 12, 43, 53 See also
this new set of Sample
Questions
Can you find the 4 energy transport
processes in this figure?
Static electricity and electric
fields (5 pts).
Like charges repel, opposite charges attract. An electric
field arrow shows the direction and strength of the force that
would be exerted on a positive charge placed at that
location. Would the electric field at Point X below, halfway
between a + and a - charge, point toward
the right, the left, or would the electric field be zero?
+ X
-
Electromagnetic radiation (10 pts).
The most important of the 4 energy transport processes
(why?). Oscillating electric and magnetic fields that can
propagate (at the speed of light) through empty space (and also
transparent materials like glass & air). Radiation can be
produced by moving charges. You add energy to cause the charges to
oscillate and produce the radiation. Energy reappears when the
resulting radiation causes electrical charges somewhere else to
move. Wavelength is one way of distinguishing between different
types of radiation (frequency is another). Would a
slowly-oscillating charge produce long- or short-wavelength
radiation? Would this be a relatively high- or low-energy form of
radiation? Electromagnetic spectrum. We will mostly be concerned
with ultraviolet (UV), visible (VIS), infrared (IR) light. What is
the wavelength interval for visible light? What is white light?
Does red light have longer, shorter, or the same wavelength as
blue light? Wavelength units.
Rules governing
the emission of radiation (10 to 15 pts). What determines how
much and what type of radiation an object will emit (the same
variable is found in both the Stefan-Boltzmann law and Wien's
law)? A light bulb connected to a dimmer switch was used to
demonstrate. Radiant energy emitted by the earth (300 K) and sun
(6000 K).
Radiative
equilibrium
(5 to 10 pts).
Energy balance. Incoming radiant energy (sunlight) is
balanced by an equal amount of (but not necessarily the same kind
of) outgoing radiant energy, temperature remains constant.
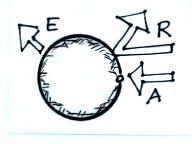 What would the relationship be between A, E, and R when this
planet is in radiative equilibrium
What would the relationship be between A, E, and R when this
planet is in radiative equilibrium
Filtering effect of the
atmosphere (10 pts). Does the atmosphere mostly absorb,
selectively absorb, or mostly transmit UV, VIS, and IR radiation?
What gases are important in each case? What does the term window
mean? What property makes water vapor, carbon dioxide, methane,
etc. greenhouse gases?
Greenhouse effect (simplified
view) (20 pts). With an
atmosphere (containing greenhouse gases), the temperature of the
earth's surface is warmer than it would be without an atmosphere.
H2O, CO2, and other greenhouse gases
selectively absorb IR radiation. The atmosphere in turn radiates
IR radiation into space and back toward the ground. How is it
possible for the earth's surface to radiate away more energy than
it receives from the sun and still be in energy balance? What
effects do clouds have on nighttime and daytime temperatures? Why?
Earth-atmosphere energy budget (15 to
20 pts). Two relatively easy questions:
(i) What percentage of the sunlight arriving at the top of the
atmosphere reaches the ground and is absorbed? (ii) What happens
to the remaining sunlight? These next questions are a little
harder: (i) Why do lower parts of the atmosphere emit more energy
downward toward the ground than upward into space? (ii) How
is it possible for the earth's surface to emit more radiant energy
than it gets from the sun? (iii) On average does the earth's
surface get more radiant energy from the sun or from the
atmosphere? How could you explain that?
Sample Questions (from the online
quiz packet)
Quiz #2: 2, 5, 7, 8,
12a,b,&c
Final Exam: 20 and see also
this set of
Sample Questions
Reviews
Mon., Mar. 10
|
4:30
- 5:30 pm
|
Haury
(Anthropology) 129
|
Tue., Mar. 11
|
4:00
- 5:00 pm |
Haury
(Anthropology) 129 |
Caution: Be
sure to put in some serious effort at understanding all of the
material on this study guide if you are planning on coming to and
remaining at one of this week's reviews.


