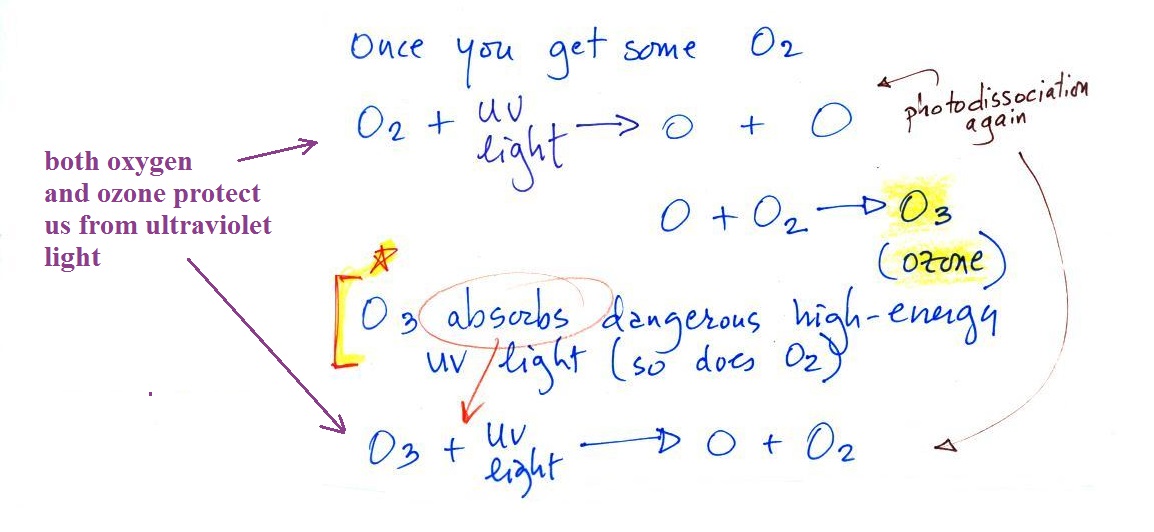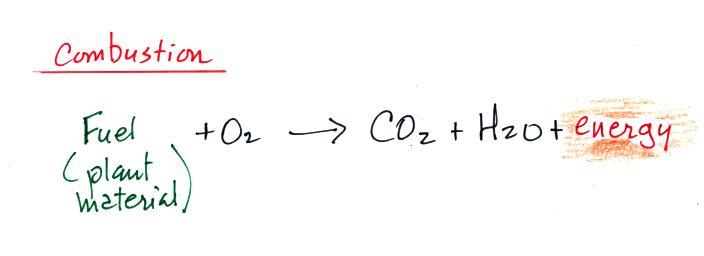Friday Jan. 12, 2018
Music from Brandi Carlile this morning: "Someday
Never Comes" (2:44), "It's Over"
(3:58), "Touching
the Ground" (3:37), "Losing Heart"
(3:35),
"The Joke"
(4:56).
The Experiment #1 materials were
checked out in class today. Try to get started on the
experiment as soon as you can. A weekend is perfect, a 3-day
weekend is even better. The experiment can take a few days
to run to completion. You should check it every hour or two
at the beginning, but it slows down as it goes and you may only
need to check it once or twice during the day toward the
end. Once you've completed the experiment return the
materials so that you can pick up a copy of the supplementary
information handout. It will help with the analysis of your
data and will make materials available for someone else that wants
to do the experiment.
Signup sheets for the remaining experiments and the Scientific
and Books reports were also passed out in class.
The first of this semester's
1S1P topics are now online. You can write reports on
as many or as few of these as you wish. I would suggest
writing at least one report just to get some idea of how they are
graded.
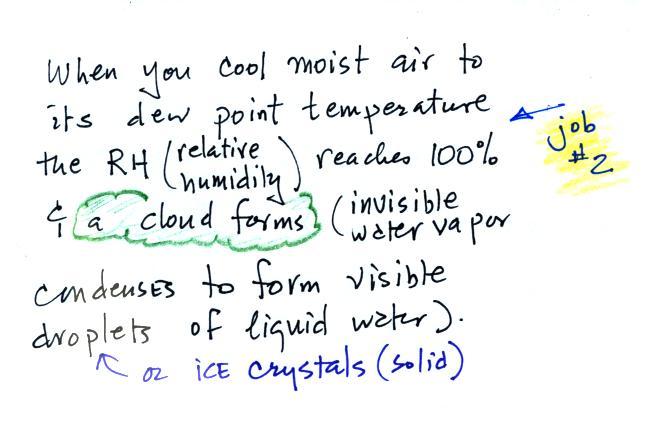
Dew point temperature continued
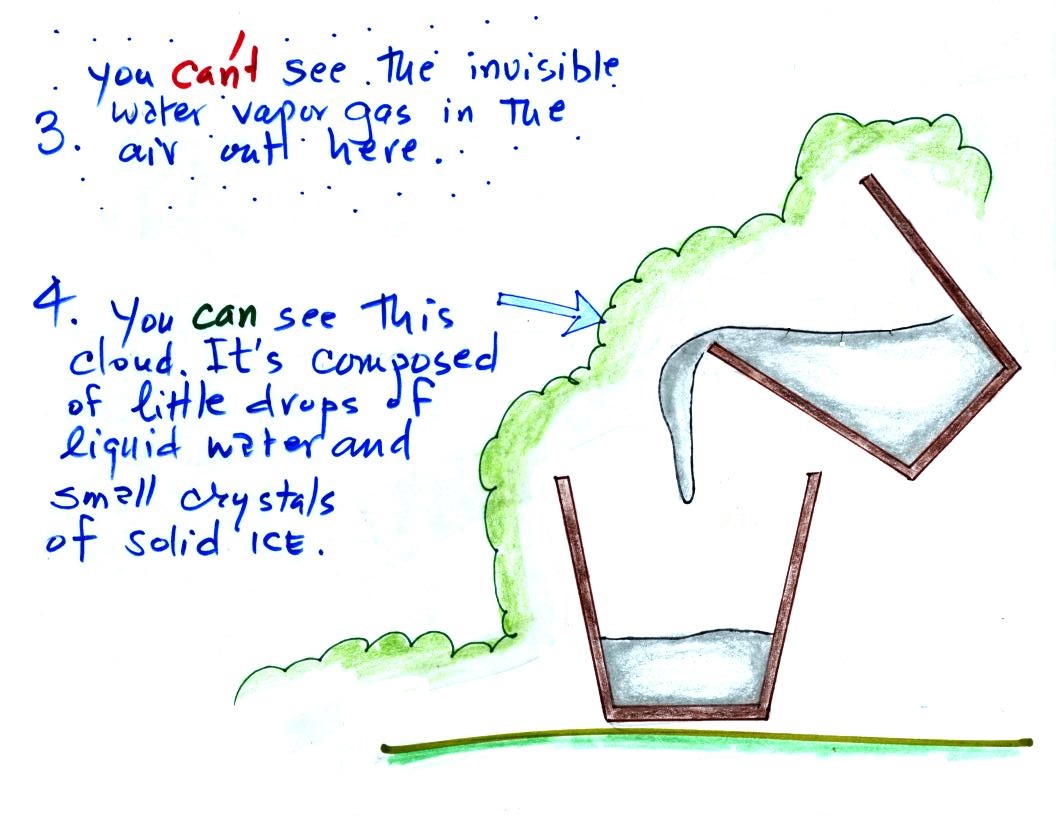
Here's a picture again of the cup of
liquid nitrogen. There are several things, both
visible and invisible, to be aware of.
First of all the
nitrogen. We can see the liquid nitrogen.
Once the liquid has evaporated and turned to gas it is
invisible. You can't see the nitrogen gas.
Another invisible gas in air, water vapor, is coming into
contact with the cold cup of liquid nitrogen. The
moist air cools enough that water vapor begins to condense
and forms a cloud consisting of very small drops of liquid
water and small crystals of ice. The cloud is visible.
We're seeing a demonstration of the dew point's "second
job."
If you cool air next to the ground to its dew point, water
vapor will condense and coat the ground (or your car) with
water. The ground will be covered with dew. If a
little thicker layer of air is cooled fog will form.
A soda bottle in the refrigerator cools to about 40 F.
In the summer in Tucson the dew point will be in the 50s or
60s. The soda bottle is cold enough that when removed
from the refrigerator it can cool the air to and below its dew
point and water vapor will condense onto the side of the
bottle as shown below. (source
of the photo)
Except for the summer, the air is usually too dry in
Tucson for this to happen. Dew points are often only in
the 20s. The 40 F soda bottle isn't able to get the air cold
enough, the relative humidity stays below 100%, and dew
doesn't form on the bottle.
A little extra information added
after class.
The air has been a little moister than normal following the
rain earlier in the week and temperatures last night were a
little cooler than normal. It didn't quite cool to the
dew point overnight but it came close. Here are some
data collected at the weather station set up near and on top
of the PAS Bldg.
The low temperature overnight was about 41F, the dew point
about 35 F. 80% relative humidity isn't high enough for
fog to form but I could see that the air was noticeably hazy
during my morning bike ride to campus.
My car was covered with dew this morning much like the soda
bottle in the picture above. The car cooled
to and maybe a little below 35 F overnight.
How to write a 1S1P report
Next we're going to cover a topic in class.
Then you'll have the option of writing a 1S1P about the same
material. We'll probably cover the subject in class a
little more quickly than I normally would. Concentrate
on how the material is organized; don't worry too much about
all the details. You might create an outline (that's
what I 'll try to do in class). That will give you an
idea of the breadth of material that you'll need to fit into
your one page report. Then, if you choose to write a
1S1P report on this topic, read through the online
reference material (the notes below are pretty nearly
identical). Focus on understanding some of the details
at that point so that you can add them to your report.
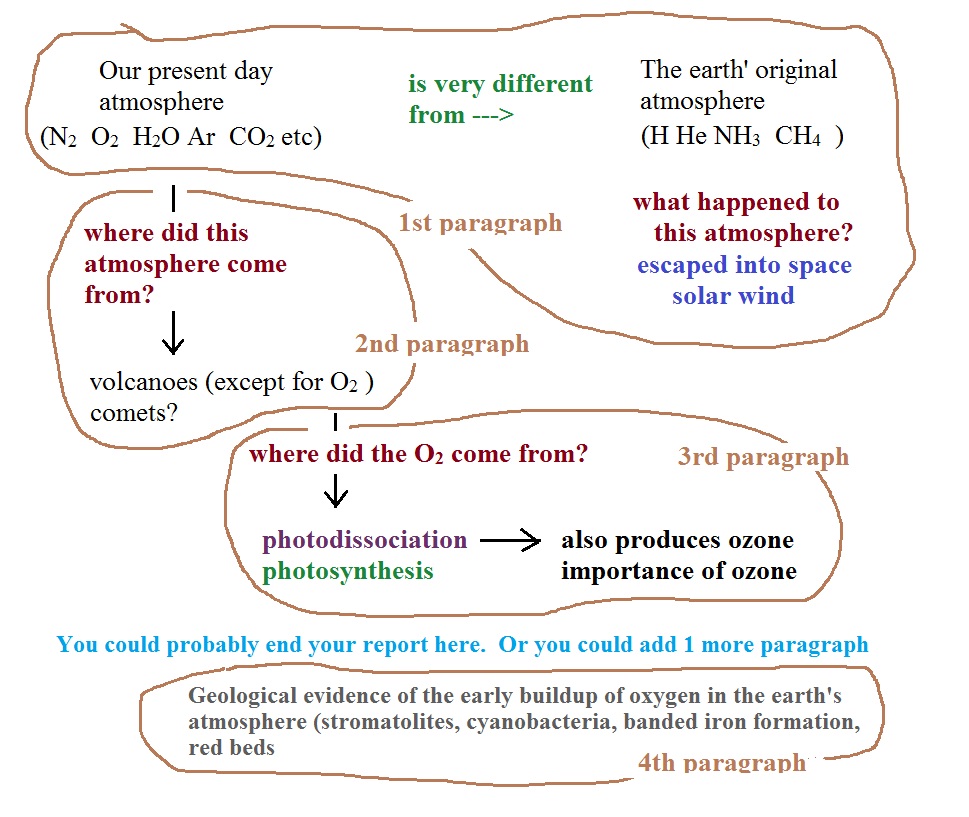
The earth's original atmosphere and our present day atmosphere
The information below wasn't presented very clearly in
class, I apologize for that. That's why we have online
notes.
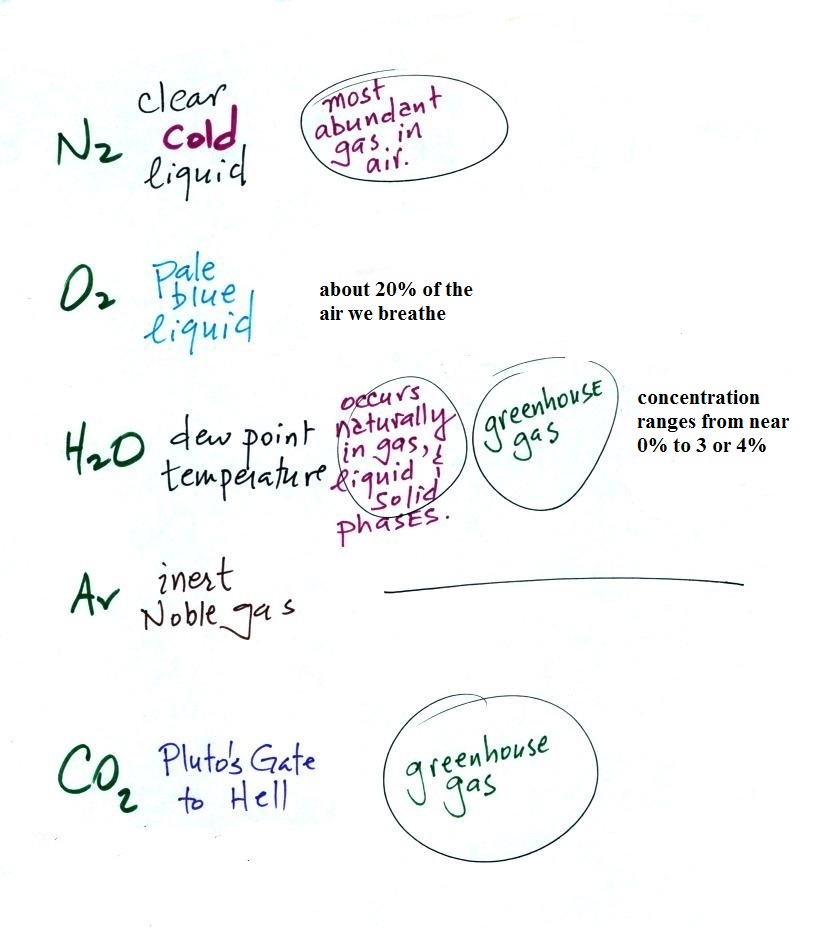
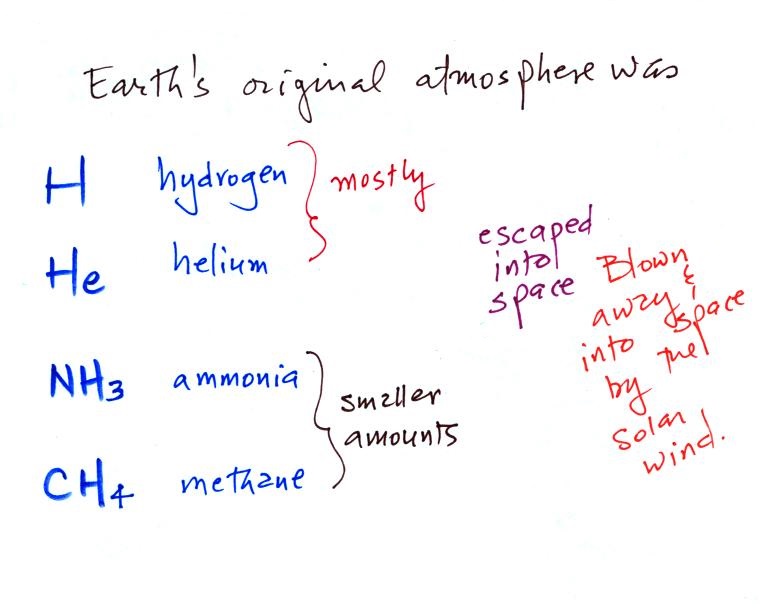
Our present day atmosphere (shown below at left) is very
different from the earth's original atmosphere (at right below)
which was mostly hydrogen and helium with lesser amounts of
ammonia and methane.
Click here to learn
more about Pluto's Gate to Hell.
The early atmosphere either escaped into space (the earth
was hot and light weight gases like hydrogen and helium were
moving around with enough speed that they could overcome the
pull of the earth's gravity) or was swept into space by the solar wind.
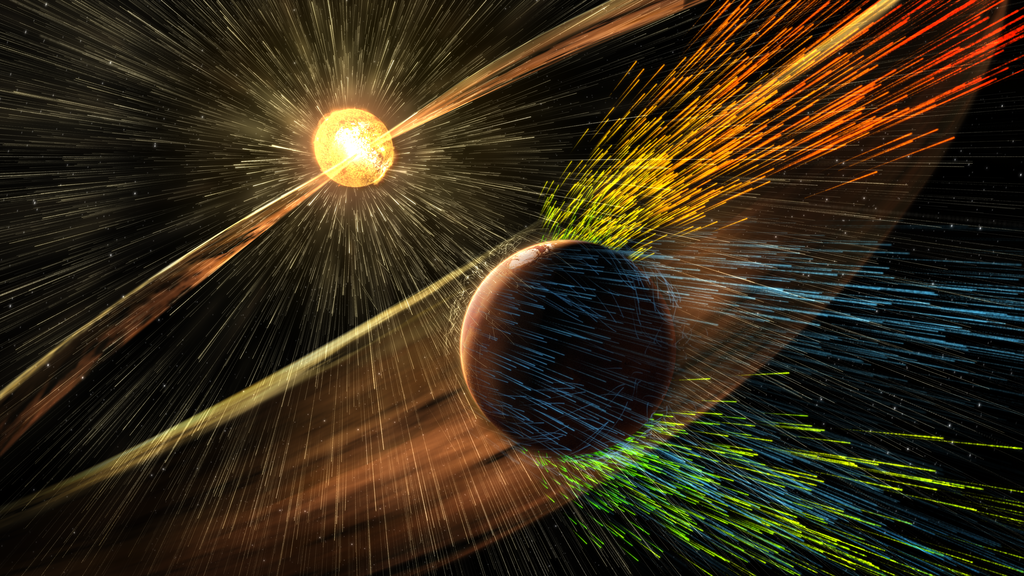
The earth's magnetic field protects our present atmosphere from
the solar wind. Mars does not have a magnetic field and
the solar wind is stripping away its thin atmosphere. The
MAVEN space mission to Mars has been measuring how quickly this
is occurring. I'm mentioning Mars mostly so that I can
insert the picture below which is an artist's impression of the
solar wind blowing away the atmosphere of Mars (source
of this image, the article also includes a solar wind animation
and more information about the MAVEN mission).
An artist's impression of the solar wind
blowing away the atmosphere of Mars (source
of this image)
Where did today's atmosphere come from?
Volcanoes
With the important exception of oxygen (and argon perhaps), most
of our present atmosphere is though to have come from volcanic
eruptions. In addition to ash, volcanoes send a lot of
water vapor, carbon dioxide, and sulfur dioxide into the
atmosphere. Carbon dioxide and water vapor are
two of the 5 main gases in our present atmosphere.
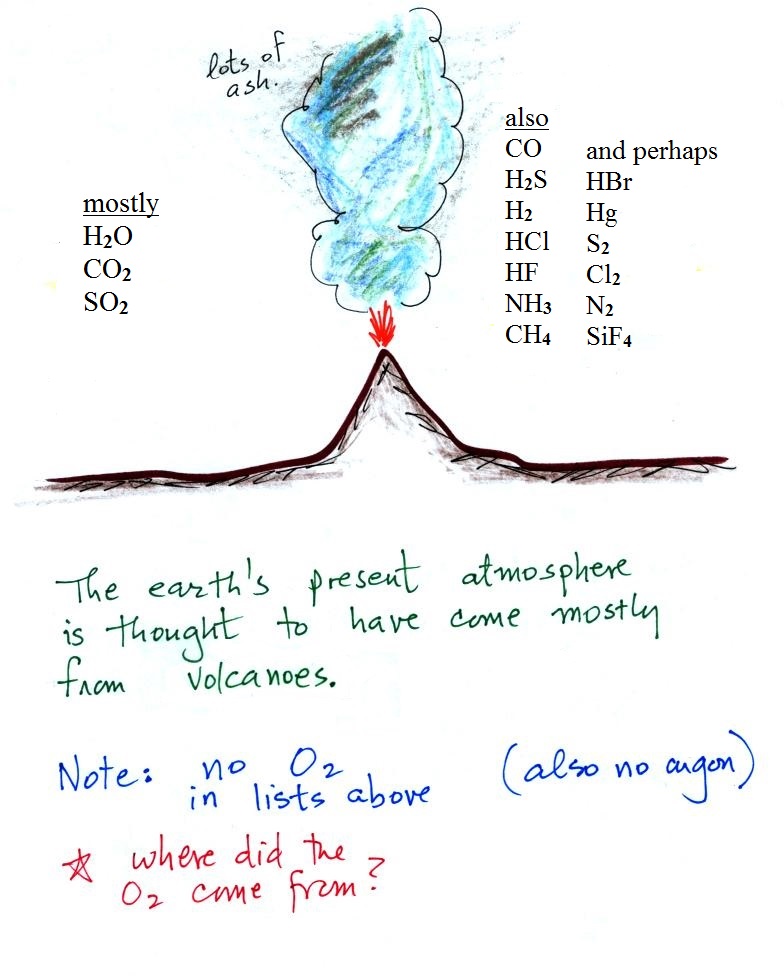
Volcanoes also emit lots of other gases, many of them are very
poisonous. Some of them are shown on the right side of
the figure. The gases in the "also" list were mentioned
in a lot of online sources, the gases in the "perhaps" list
were mentioned less frequently. The relative amounts of
these "also" and "perhaps" gases seems to depend a lot on
volcano type.
As the earth began to cool the water vapor condensed and
began to create and fill the earth's oceans. Carbon
dioxide dissolved in the oceans and was slowly turned into
rock. Nitrogen containing compounds like ammonia (
NH3 ) and
molecular nitrogen ( N2 ) are also emitted by
volcanoes. I'm guessing that the nitrogen in NH3
reacted with other gases
to produce N2.
Molecular nitrogen is pretty nonreactive so
once in the air its concentration was able to built up over
time.
The argon found in air apparently comes from the
radioactive decay of potassium in the ground. Three isotopes of
potassium occur naturally: potassium-39 and potassium-41 are
stable, potassium-40 is radioactive and is the source of the
argon in the atmosphere.

The photo above shows the
Eyjafjallajokull volcano in Iceland photographed on Apr. 17,
2010 (image
source)
(Here
are some additional
pictures of the Eyjafjallajökull
volcano that you should really have a look at.
Eyjafjallajökull
caused severe disruption of airline travel between the
US and Europe.
Here's
another
set of photos also from the Boston Globe)
Comets
This photograph is a mosaic of 4 images taken over a
roughly 20 minute period from an altitude of 28.6 km
(17.8 miles). Photo credit: "Comet 67P on 19
September 2014 NavCam mosaic" by ESA/Rosetta/NAVCAM
found a
Wikipedia article on the Rosetta mission.
|
A mosaic of the first two images showing the Philae
lander on the comet's surface. photo credit:
ESA/Rosetta/Philae/CIVA photo source
|
These photographs of Comet 67P/Churyumov-Gerasimenko taken
by the European Space Agency Rosetta spacecraft. The
spacecraft was launched on March 2, 2004 and went into orbit
around the comet on August 6, 2014. On November 12, the
Rosetta spacecraft deployed the Philae lander which
successfully landed on the surface of the comet and operated
for a brief time. How amazing is that? The lander
was not able to fully deploy its solar panels and used up its
battery power and went into "sleep" mode after about 60 hours
of operation. In June 2015 the comet had moved
into a sunnier part of its orbit and the lander
began sending data again. When the comet had
reached perihelion (the shortest distance between the comet
and the sun), the Rosetta spacecraft photographed material
being ejected from the comet (you'll find a nice animation here).
I've included this photograph of a comet because some
researchers don't believe that volcanic activity
alone would have been able to account for all the water that
is on the earth (oceans cover about 2/3rds of the earth's
surface). They believe that
comets and asteroids colliding with the earth may have brought
significant amounts of water (you can read more about the
composition of comets at https://www.universetoday.com/40692/what-are-comets-made-of/
). The Rosetta spacecraft has determined that the water
on this particular comet differs from the composition of the
water in the earth's oceans. This
reference reports "The ratio of deuterium to hydrogen in the water from the comet
was determined to be three times that found for terrestrial
water." Deuterium is a hydrogen isotope, the nucleus
contains one proton and one neutron; the nucleus of "normal"
hydrogen just contains a proton. This suggests that
comets like 67P anyways were probably not an important source
of the earth's water.
The Lunar and Planetary Laboratory here at the U of A is one
of the lead teams on the ORISIS - REx, mission
which was launched in Fall 2016. The goal of that
mission is to send a spacecraft to Bennu, a near-earth
asteroid, collect a sample of the asteroid and return it to
earth. If all goes according to plan the spacecraft will
rendezvous with the asteroid in Aug. 2018 with sample
acquisition occurring in July 2020. The return capsule,
containing the sample from the asteroid, should land in Utah
on Sept. 24, 2023.
Where did the oxygen in
our atmosphere come from?
Oxygen is in H2O, CO2,
and SO2 (and many of the other gases emitted by
volcanoes) but volcanoes don't emit molecular oxygen (
O2 ) the stuff we need in
order to survive. Where did the
O2 come from? There
are a couple of answers to that question.
1. Photodissociation
Oxygen is thought to have come from
photo-dissociation of water vapor and carbon dioxide by
ultraviolet (UV) light (the high energy UV light is able to split
the H20 and CO2 molecules into pieces). Two of the pieces,
O and OH, then react to form O2 and H.
By the way I don't expect you to remember the chemical formulas
in the example above. It's often easier and clearer to show
what is happening in a chemical formula than to write it out in
words. If I were to write the equations down, however, you
should be able to interpret them. Ultraviolet is a
dangerous, high energy, potentially deadly form of light and it's
probably also good to remember that ultraviolet light is capable
of breaking molecules apart.
Production and destruction of ozone
Once molecular oxygen (O2)
begins to accumulate in the air ultraviolet (UV) light in sunlight
can split the O2 apart to make atomic oxygen (O). The atoms
of oxygen can react with molecular oxygen to form ozone (O3). This is shown in the top
part of the figure below.
This is an important step. Ozone in the upper
atmosphere began to absorb the dangerous and deadly forms
ultraviolet light and prevented it from reaching the
surface. Life forms could then begin to safely move from
the oceans onto land (prior to the buildup of ozone, the ocean
water offered protection from UV light). The absorption of
UV light by ozone is shown at the bottom of the figure above and
repeated below
O3 + UV light ---> O2 + O
You might think the two "pieces" O2 and O would
recombine. But if you picture hitting something with a
hammer and break it, the pieces usually fly off in different
directions. That's essentially what happens with the O and
O2 .
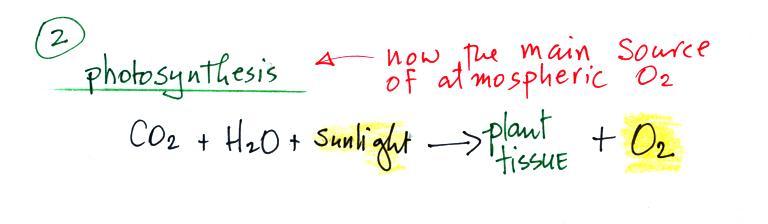
2. Photosynthesis
Once plant life had developed sufficiently and once plants had
moved from the oceans onto land, photosynthesis became the
main source of atmospheric oxygen.
Photosynthesis in its most basic form is shown in the chemical
equation above. Plants need water, carbon dioxide, and
sunlight in order to grow. They can turn can turn H20 and CO2 into plant material. Photosynthesis
releases oxygen as a by product.
Combustion is essentially just the opposite of photosynthesis and
is shown below.
We burn fossil fuels (dead but undecayed plant material) to
generate energy. Water vapor and carbon dioxide are by
products. Combustion is a source of atmospheric
CO2
(photosynthesis is a "sink" for atmospheric CO2 , it removes CO2 from the air).
Carbon dioxide will probably be the subject of an upcoming 1S1P
assignment and we'll see these two equations again there and when
we study the greenhouse effect and global warming.
This is also in its most basic form what we (people) are
doing. We eat food (fuel) and breathe in oxygen. The
food is "burned" to generate the energy needed to keep our bodies
functioning, we exhale carbon dioxide.
This is as far as we got in class
today but we did cover the main points that should
go into a 1S1P report on the Origin and Evolution of the Earth's
atmosphere.
Here's a cleaner version of the outline of what we
covered today:
Add a few details and some explanation to this outline,
turn it all into sentences and paragraphs and you'll have a
pretty good report.
We'll cover the remaining material
at the start of class next Wednesday.
Geological evidence
of early oxygen in the earth's oceans and atmosphere
The
following figure is the first page in the packet of
photocopied ClassNotes.
This somewhat confusing figure shows some of the important
events in the history of the earth and evolution of the
atmosphere. There are 5 main points I want you to take from
this figure. Points 1 - 3 are the most important.
First, Point 1: the
earth is thought to be between 4.5 and 4.6 billion years
old. If you want to remember the earth is a few billion
years old that is probably close enough. A relatively
minor point shown in the figure: the formation of the earth's
molten iron core was important because it gave the earth a
magnetic field. The magnetic field is what deflects the
solar wind and prevents the solar wind from blowing away our
present day atmosphere.
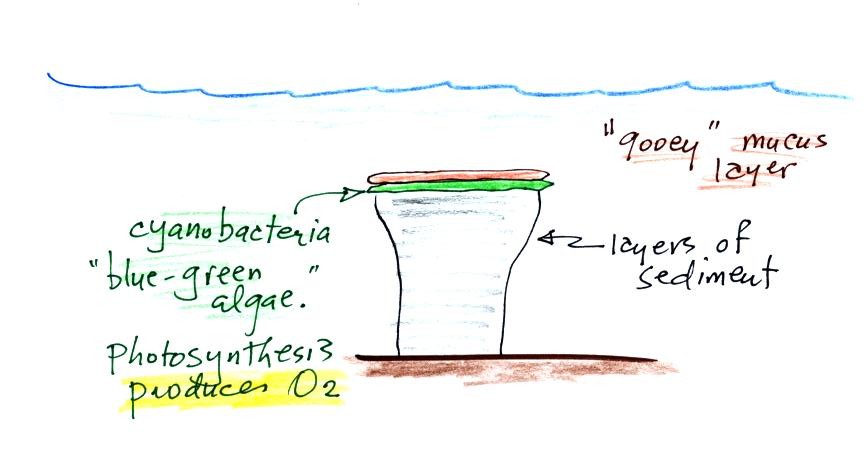
Stromatolites (Point
2) are geological features, column-shaped structures
made up of layers of sedimentary rock, that are
created by microorganisms living at the top of the
stromatolite (I'm not a geologist and I've never actually seen
a stromatolite, so this is all based on photographs and
written descriptions). Fossils of the very small
microbes (cyanobacteria = blue green algae) have been found in
stromatolites as old as 2.7 B years and are some of the
earliest records of life on earth. Much older (3.5 to
3.8 B year old) stromatolites presumably also produced by
microbes, but without microbe fossils, have also been
found.
Stromatolites
Blue green algae grows at the top of the column, under water
but near the ocean surface where it can absorb sunlight. As
sediments begin to settle and accumulate on top of the algae they
start to block the sunlight. The cyanobacteria would then
move to the top of this sediment layer and the process would
repeat itself. In this way the stromatolite column would
grow layer by layer over time.
You might be wondering why we are learning about
stromatolites. They're evidence of an early form of
life on earth being able to produce oxygen using photosynthesis.

|

|
Living stromatolites are found in
a few locations today.The two pictures above are from Lake
Thetis (left) and Shark
Bay (right) in Western Australia (the two
photos above and the photograph below come from this source).
The picture was probably taken at low tide, the stromatolites
would normally be covered with ocean water. It doesn't look
like a good place to go swimming, I would expect the top surfaces
of these stromatolites to be slimy (some forms of blue green algae
may be toxic). Hamelin Pool in Western Australia is a World
Heritage Area, the stromatolites there are the oldest and largest
living fossils on earth (see this
source for more information)
Living stromatolites at Highborne Cay in the Bahamas.
Banded iron formation
Point 3 refers to the banded iron formation, a type of rock
formation. These rocks are 2 - 3 billion years old (maybe
older) and are evidence of oxygen being produced in the earth's
oceans. Here are pictures of the two samples of banded iron
formation rock that I passed around in class.
The main
thing to notice are the alternating bands of red and
black. The rocks are also relatively heavy
because they contain a lot of iron. The next
paragraph and figure explain how these rocks formed.
Rain would first of all wash iron
ions from the earth's land surface into the ocean
(this was at a time before there was any oxygen in the
atmosphere). Once in the ocean, the iron ions
reacted with oxygen from the cyanobacteria living in
the ocean water to form hematite or magnetite.
These two minerals precipitated out of the water to
form a layer on the sea bed. This is what
produced the black layers.
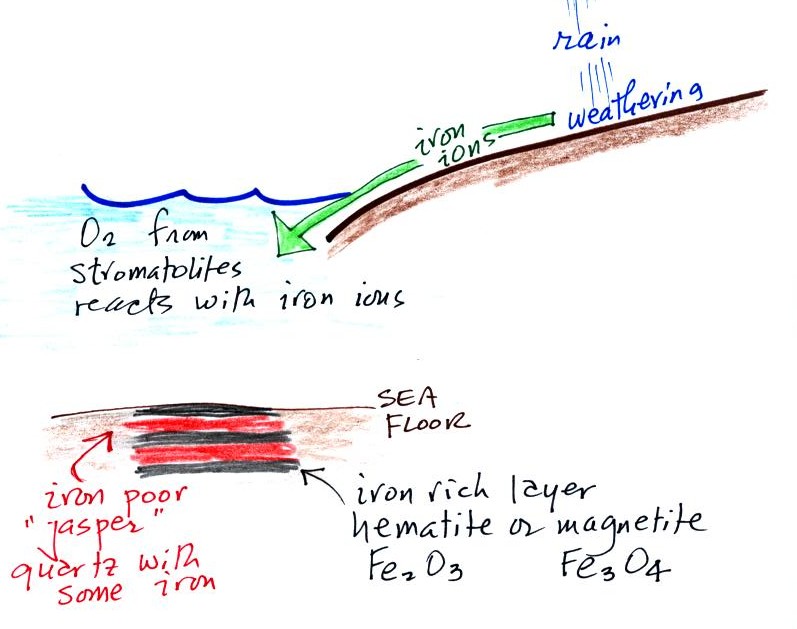
Periodically the oxygen production would
decrease or stop (rising oxygen levels might have killed the
cyanobacteria or seasonal changes in incoming sunlight might
have slowed the photosynthesis). During these times of low
oxygen concentration, red layers of jasper would form on the
ocean bottom. The jasper doesn't contain as much
iron.
Eventually the cyanobacteria would recover, would begin
producing oxygen again, and a new layer of hematite or magnetite
would form. The rocks that resulted, containing
alternating layers of black hematite or magnetite and red layers
of jasper are known as the banded iron formation. In addition to the red and black layers,
you see yellow layers made of fibers of quartz in the samples
passed around class.
Red beds
Eventually the oxygen in the oceans reacted with all of the
iron ions in the water. Oxygen was then free to diffuse from the ocean
into the atmosphere. Once in the air, the oxygen could react
with iron in sediments on the earth's surface. This produced
red colored (rust colored) sedimentary rock. These are
called "Red Beds" (Point 4).
None of these so-called red beds are older than about 2 B years
old. Thus it appears that a real buildup up of oxygen in the
atmosphere began around 2 B years ago.
Red Rock State Park near
Sedona Arizona (an example of "red beds" that formed during the
Permian period 250-300 million years
ago)
Oxygen concentrations reached levels that are about the same as
today around 500 to 600 million years ago (Point 5 in the figure).