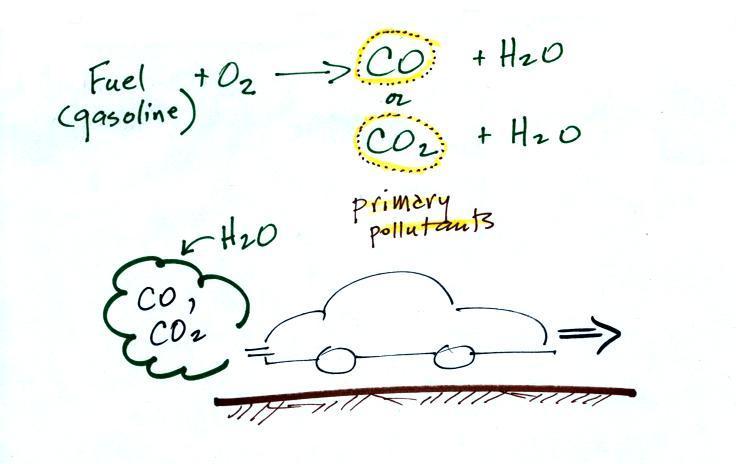
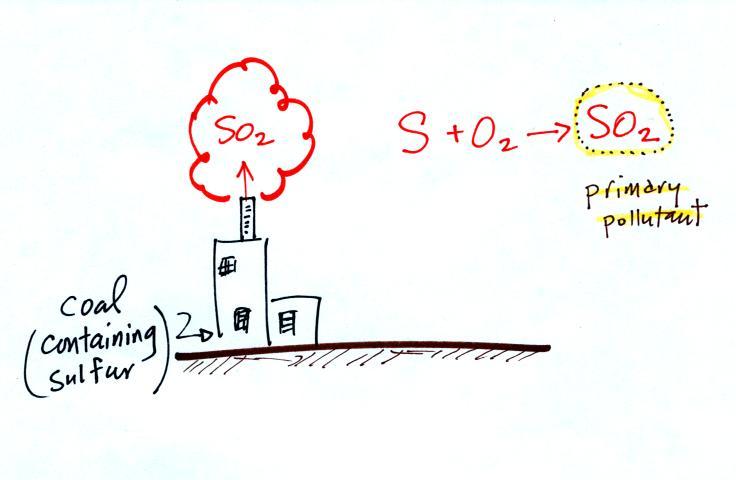
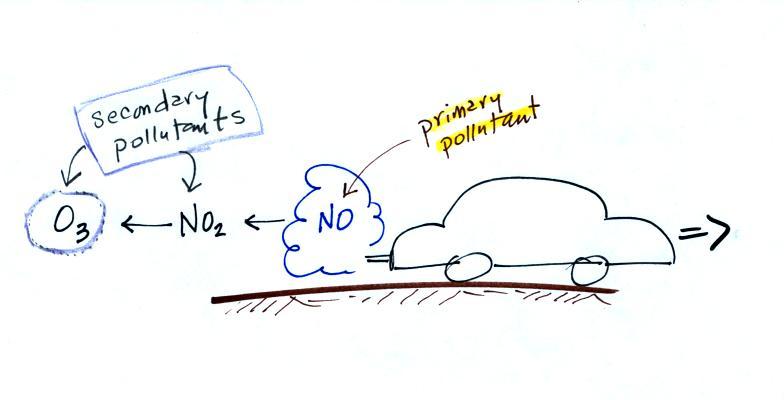
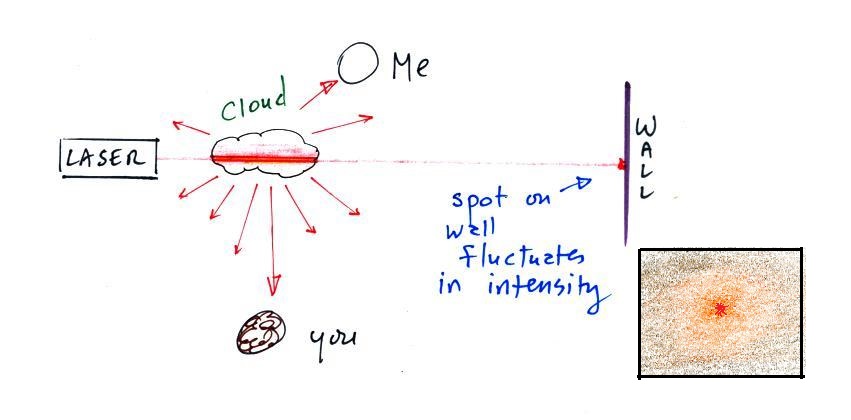
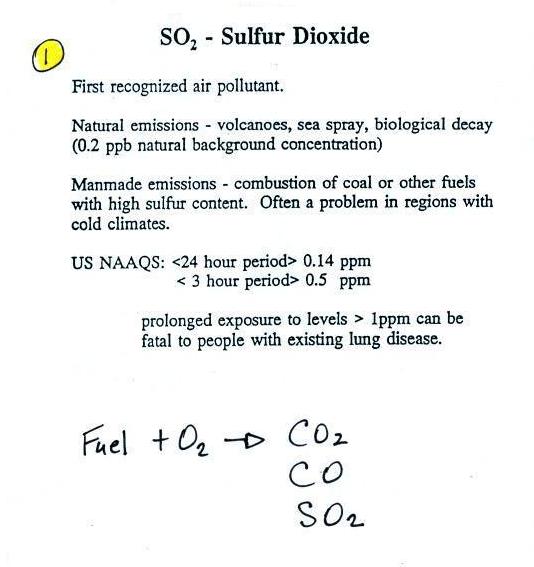
 |
|
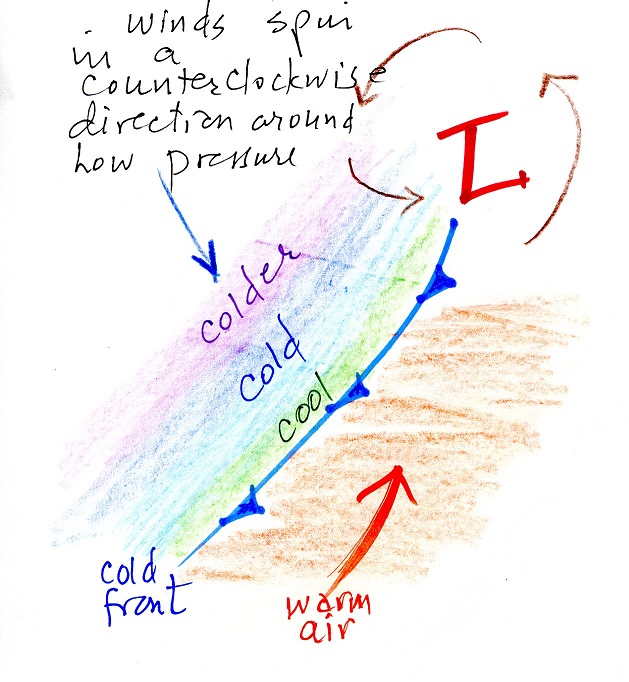
| The "bull's eye" center of low pressure in
the upper left hand corner of this photograph is the center of the storm. Winds blowing on the western side of the storm move cold air southward. A cold front indicates the front edge of this advancing cold air. The front will pass through Arizona sometime late Friday or during the day on Saturday |
Top view of a cold front. You would expect to see warm winds blowing from the south or southwest before the front passes through and colder winds from the west or northwest once the front moves through. Gusty winds often accompany passage of the front. The coldest temperature may occur a day or two after the front has passed through. |

 |
 |
 |
 |

 |
 photo by Jessie
Eastland
|





 The caption to this
photo from The Guardian reads
"Arsenal goalkeeper Jack Kelsey peers into the fog. The 'smog' was so thick the game was eventually stopped." |
 The smog in this photo is the thickest I was able to find. Visibility here is perhaps 10 or 20 feet. (source of this image) |
 Buses had to creep along to avoid
hitting someone or something.
from: http://news.bbc.co.uk/1/hi/health/2545747.stm |
 Someone would often walk out ahead
of a bus to be sure the way was clear.
from: http://news.bbc.co.uk/1/hi/england/2543875.stm |
 You can get a feel for the cause of
the smog
in this photograph by Paul Lowry in an article in SAGEMagazine. |
 |

