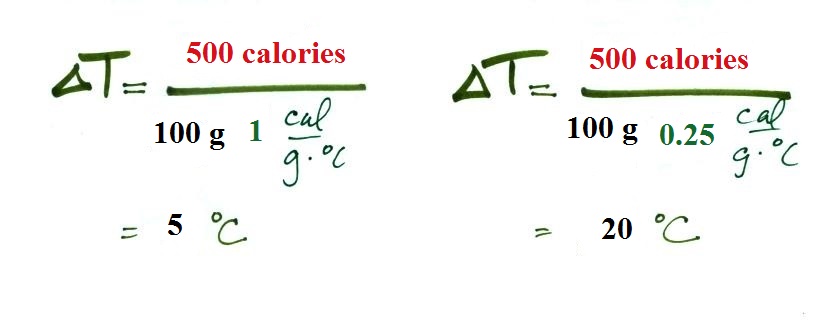The atoms or
molecules inside the warmer object will be moving more
rapidly (they'll be moving freely in a gas, just
"jiggling" around while still bonded to each other in
a solid). Since kinetic energy is energy
of motion, temperature gives you an idea of the
average speed of the moving atoms or molecules in a
material.
You need to be careful what temperature scale
you use when using temperature as a measure of average
kinetic energy. You must use the Kelvin
temperature scale because it does not go below zero (0
K is known as absolute zero). The smallest amount of
kinetic energy you can have is zero kinetic
energy. There is no such thing as negative
kinetic energy.
There
are three temperature scales that we might have
occasion to use in this class. They're
shown below. There are two temperatures that
you should try to remember on each scale.
The boiling and freezing points of water
on both the Celsius and the Fahrenheit scales (the freezing
point of water and the melting point of ice are the
same). Remember that the Kelvin scale doesn't go below
zero. 0 K is referred to as absolute zero, it's as cold
as you can get. A nice round number of the average
temperature of the earth is 300 K, that's the last temperature
value to remember.
Here's some additional temperature data that I'm including
just in case you're interested.

You certainly don't need to try to
remember all these numbers. The world high temperature
record value of 136 F above was measured in Libya at a
location that was only about 35 miles from the Mediterranean
coast. Water, as we will see, moderates climate, it
reduces the extremes, so it seems odd that such a high
temperature would have been recorded there. The World
Meteorological Organization recently decided the 136 F
reading was invalid and the new world record is the 134 F
measurement made in Death Valley. There is also some
question about the 134 F Death Valley value (see this
article in Wikipedia). There seems to be some
agreement that 129 F is the highest reliable measurement of
temperature. Temperatures that hot have been measured
at multiple locations.
The continental US cold temperature record of -70 F was
set in Montana and the -80 F value in Alaska. The
world record -129 F was measured at Vostok station in
Antarctica. This unusually cold reading was the result
of three factors: high latitude, high altitude, and location
in the middle of land rather than being near or surrounded
by ocean (again water moderates climate, both hot and
cold).
Liquid nitrogen is very cold but it is still quite a bit
warmer than absolute zero. Liquid helium gets within a
few degrees of absolute zero, but it's expensive and there's
only a limited amount of helium available. So I would
feel guilty bringing some to class; plus I don't think it
would look any different than liquid nitrogen.
4. Energy, temperature, and specific heat
When you add energy to an object, the object will usually
warm up (or if you take energy from an object the object will
cool). It is relatively easy to come up with an equation
that allows you to figure out what the temperature change will
be (this is another equation I'll try to remember to write on
the board before the next quiz. Try to understand
it, you don't have to memorize it.
The temperature change, ΔT, will first depend on how much
energy was added, ΔE. This is a
direct proportionality, so ΔE is in the
numerator of the equation (ΔE and ΔT
are both positive when energy is added, negative when energy is
removed)
When you add equal amounts of energy to large and small pans
of water, the water in the small pan will get hotter. The
temperature change, ΔT, will depend on
the amount of water, the mass. A small mass will mean a
large ΔT, so mass should go in the
denominator of the equation.
Specific heat is what we use to account for the fact that
different materials react differently when energy is added to
them. A material with a large specific heat will warm more
slowly than a material with a small specific heat.
Specific heat has the same kind of effect on ΔT
as mass. Specific heat is sometimes called "thermal mass"
or "thermal capacity."
Here's an important example that will show the effect of
specific heat (see page
45b in the ClassNotes).
Equal amounts of energy (500 calories) are added
to equal masses (100 grams) of water and soil. We use
water and soil in the example because most of the earth's
surface is either ocean or land. Before we do the calculation,
try to guess which material will warm up the most.
Everything is the same except for the specific heats. Will
water, with its 4 times larger specific heat, warm up more or
less than the soil?
The details of the calculation are shown below.
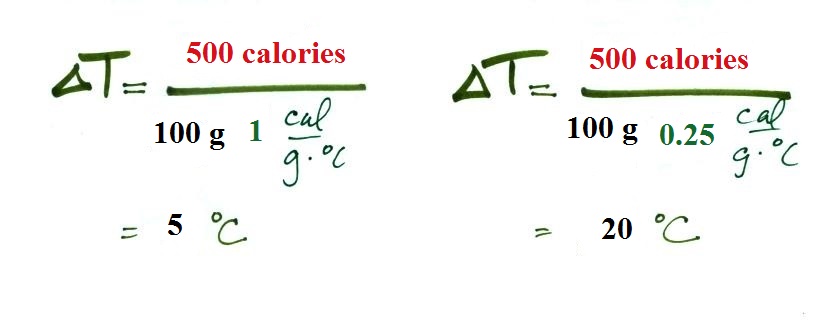
With its higher specific heat, the water doesn't heat up
nearly as much as the soil. If we had been removing
energy the water wouldn't cool off as much as the soil
would.
5. Water moderates climate
These different rates of warming of water and soil have
important effects on regional climate.
Oceans moderate the climate. Cities near a large body
of water won't warm as much in the summer and won't cool as much
during the winter compared to a city that is surrounded by land.
Water's ΔT is smaller than land's because water
has a higher specific heat.
The yearly high and low monthly average temperatures are
shown at two locations above. The city on the coast has a
30o F annual range of temperature (range is
the difference between the summer and winter
temperatures). The city further inland (assumed to be at
the same latitude and altitude) has an annual range of
60o F. Note that both cities have the same 60o
F annual average temperature.
Water moderates climates - it reduces the difference between
summertime high and wintertime low temperatures.
Growing tomatoes in the desert -
practical application
Here's another situation where you can take
advantage of water's high specific heat to moderate climate on a
smaller scale (it fits better in the Spring semester edition of
the class than the Fall semester).
You need to start tomatoes
early in Tucson (mid February), so that they can produce fruit
before it gets too hot. I usually start mine in February
and you need to protect the plants from frost.
Here's one way of doing that. You
moderate the climate and surround each plant with a "wall o
water" - a teepee like arrangement that surrounds
each plant. The cylinders are filled with water and they
take advantage of the high specific heat of water and won't cool
as much as the air or soil would during a cold night. The
walls of water produce a warm moist micro climate that the
tomato seedlings love. The plastic is transparent so
plenty of sunlight can get through. Note the brocolli
growing in the background, it isn't nearly as sensitive to the
cold and doesn't require protection.
6. Energy
transport processes