April 3, 2008
These notes were put together in a
hurry on Friday afternoon. They haven't yet been carefully
proofread and may contain a lot of typographical errors.

Extra office hours continue through the rest of this week.
The answers to the
Controls of Temperature Optional Assignment are available online.
The graded papers were returned in class today.
The 3rd (and final) 1S1P Assignment is now
available.
The humidity Optional Assignment was collected in class today.
The graded papers will be returned next Tuesday. A handout with
answers to the questions was distributed in class so that you won't
have to wait until next week to begin preparing for next week's quiz.
Speaking of the quiz, the Quiz #3 Study Guide
is now in its final form. There were a couple of short sections
added to the beginning of the preliminary version of the study guide.
I forgot to mention that Expt. #4 is due next Tuesday and forgot to
remind you to bring your experiment materials to my office. I
will probably extend the deadline until next Thursday so that I can
make an announcement in class next Tuesday.
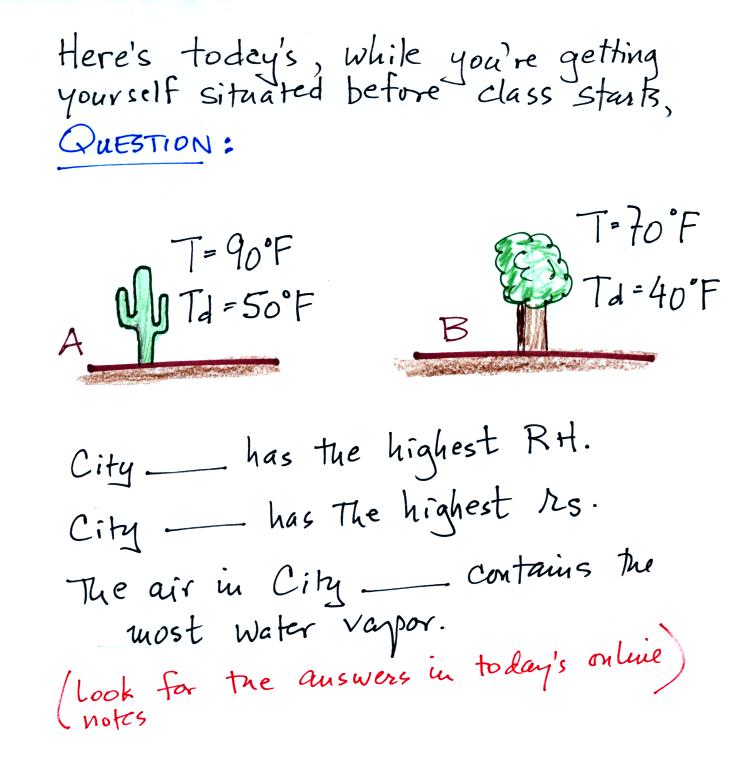
Click here
after you have thought about this for awhile.
Here's another question that the other class will see on
Friday. Click here to see the answer.
Because we
have just finished a section on clouds, we will spend a little time
looking at satellite photographs and what they tell you about the
clouds they view. You'll
find satellite photographs discussed on pps
99-100 in the photocopied class notes (also in the text: pps
240-243 (Chap. 9) in
the 5th eds of the text & pps 236-240 in the 4th edition of the text).
A handout with most of the following figures was distributed in
class. Extra copies should be available in class next Tuesday.
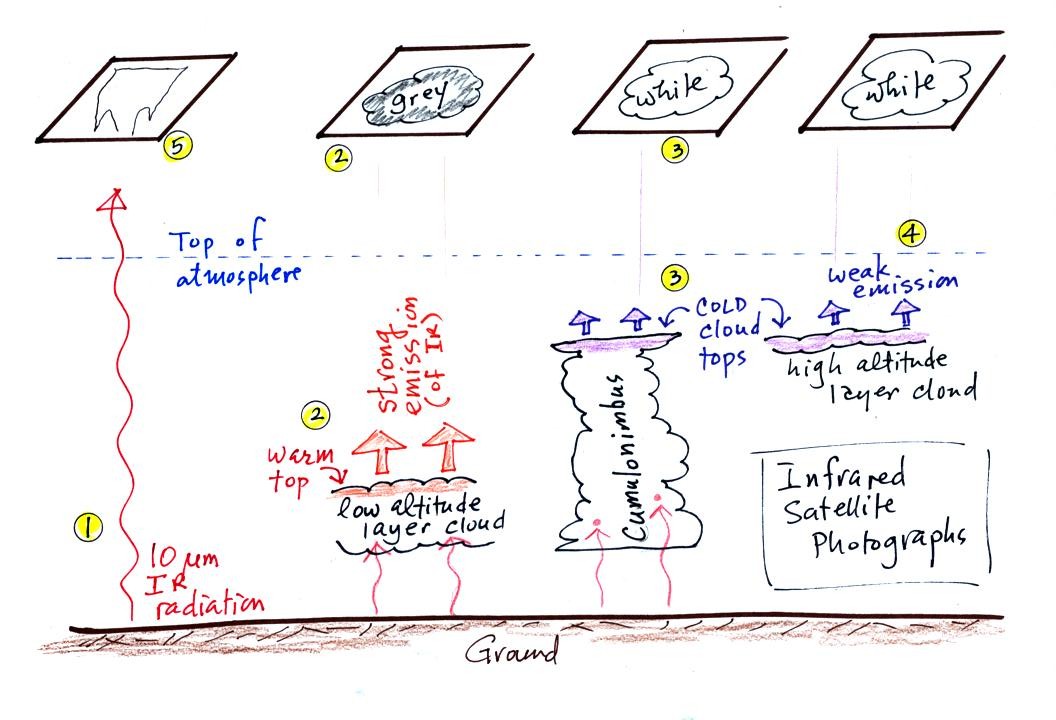
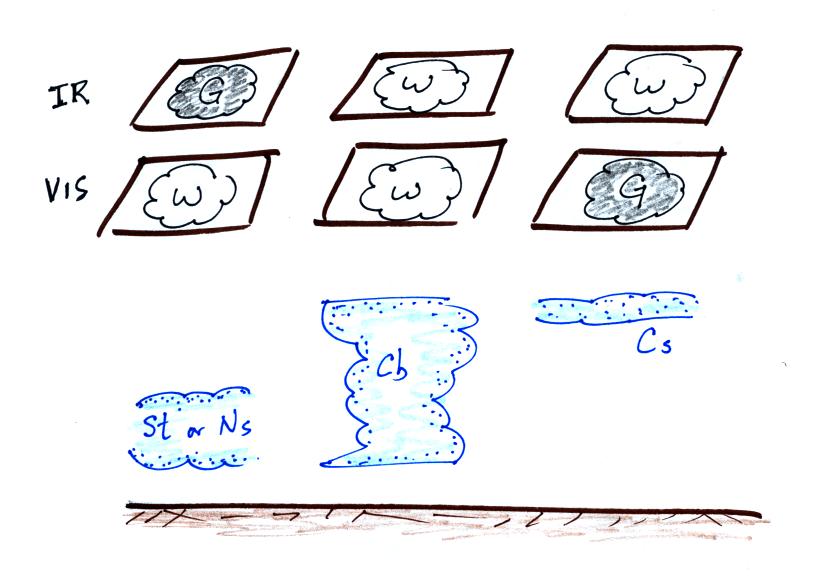
1. An infrared satellite photograph detects the
10 um IR
radiation
actually
emitted by the ground or by clouds. You don't depend on seeing
reflected
sunlight, so clouds can be photographed during the day and at
night. You may recall that 10 um radiation is in the middle of
the atmospheric window, so this radiation is able to pass through air
without being absorbed.
2. Clouds absorb 10 um radiation and then reemit
radiation. The top surface of a low altitude cloud will be
relatively warm. Warmer objects emit IR radiation at a greater
rate or at higher intensity (the Stefan Boltzmann law from Chap.
2).
This is shown as grey on an IR satellite photograph. A
grey unimpressive looking cloud on an IR
satellite photograph may actually be a thick nimbostratus cloud that is
producing a lot of rain or snow.
3. Cloud tops found at high altitude are cold and emit IR
radiation at a lower rate or at lower intensity. This shows up
white on an IR photograph.
4. Two very different clouds (a thunderstorm and a
cirrostratus cloud) would both appear white on the satellite photograph
and would be difficult to distinquish. Meteorologists are
interested in locating tall thunderstorms as they can produce severe
weather.
5. The ground changes temperature during the course of the
day. On an infrared satellite animation you can watch the ground
change from black (afternoon when
the ground is warmest) to grey (early morning when the ground is cold)
during the course of a day. The ocean right alongside doesn't
change temperature much during the day and remains grey throughout the
day.

A visible satellite photograph photographs sunlight that is
reflected
by clouds. You won't see much on a visible satellite photograph
at night. Thick clouds are good reflectors and appear
white. Thinner clouds don't reflect as much light and appear
grey. The low altitude layer cloud and the thunderstorm would
both appear white on this photograph and would be difficult to
distinquish.
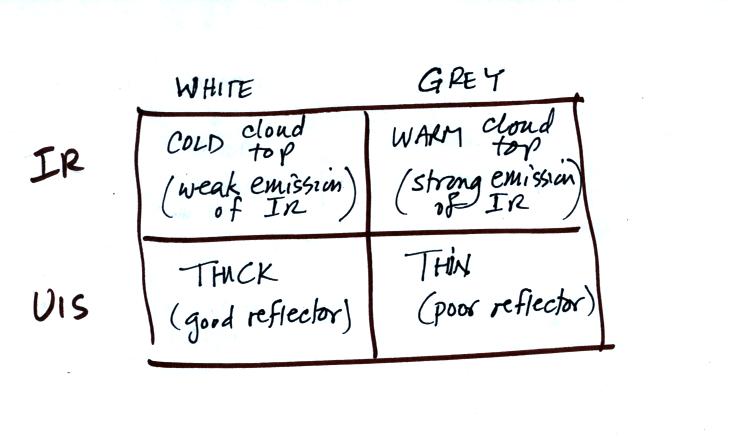
Here's a summary of what we have learned so far.
The figure below shows how
if you combine both visible and IR
photographs you can begin to distinquish between different types of
clouds.
There is one more type of satellite image worth mentioned, a
water
vapor image.
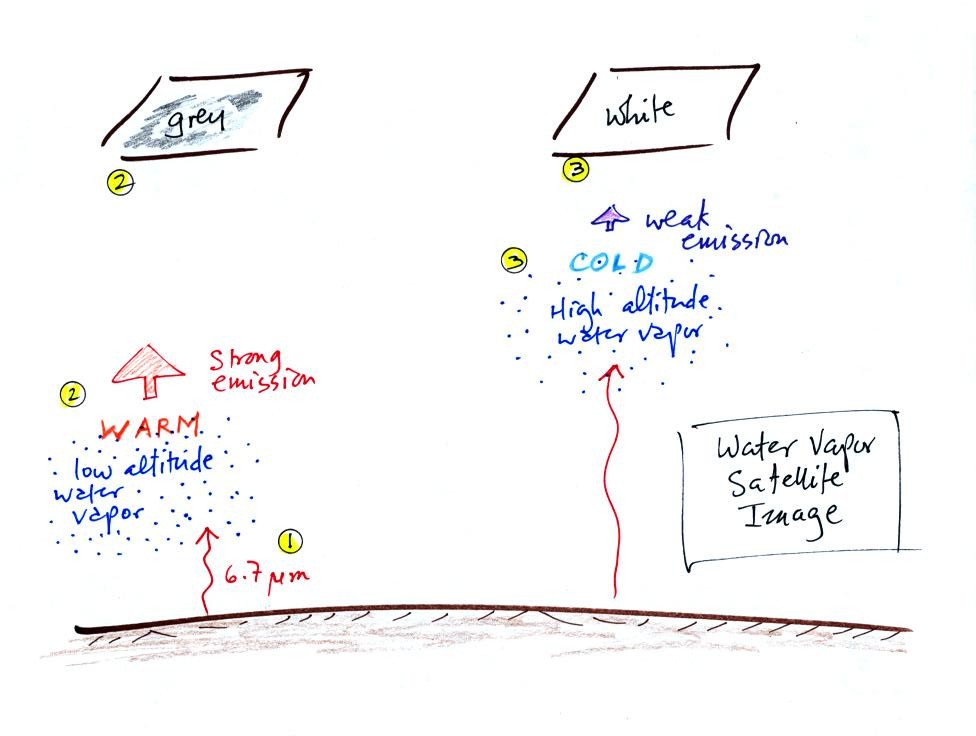
This is also a type of IR photograph. It detects a
different
wavelength of IR radiation. 6.7 um radiation is absorbed and
emitted by water vapor in the atmosphere. Warm low altitude water
vapor appears grey and unimpressive. Higher altitude water vapor
appears white on the satellite photograph. But
remember the high altitude
air is cold and there isn't much water vapor up there. The
utility of these photographs is not to show you whether a lot of
moisture is moving into an area but rather they reveal wind motions in
regions where there aren't clouds.
We were
able in class on Thursday to get started on the next
topic: formation of
precipitation. It is not as easy to make precipitation as you
might think. Only nimbostratus and cumulonimbus clouds are able
to do it.
This figure shows typical sizes of cloud
condensation nuclei (CCN), cloud droplets, and raindrops (a human hair
is about 50 um thick for comparison). As we
saw in the cloud in a bottle demonstration it is relatively easy to
make cloud droplets. You cool moist air to the dew point and
raise the RH to 100%. Water vapor
condenses pretty much instantaneously onto a cloud condensation nucleus
to form a cloud droplet. It
would take much longer (a day or more) for condensation to turn a cloud
droplet
into a
raindrop. You know from personal experience that once a cloud
forms you don't have to wait that long for precipitation to begin to
fall.
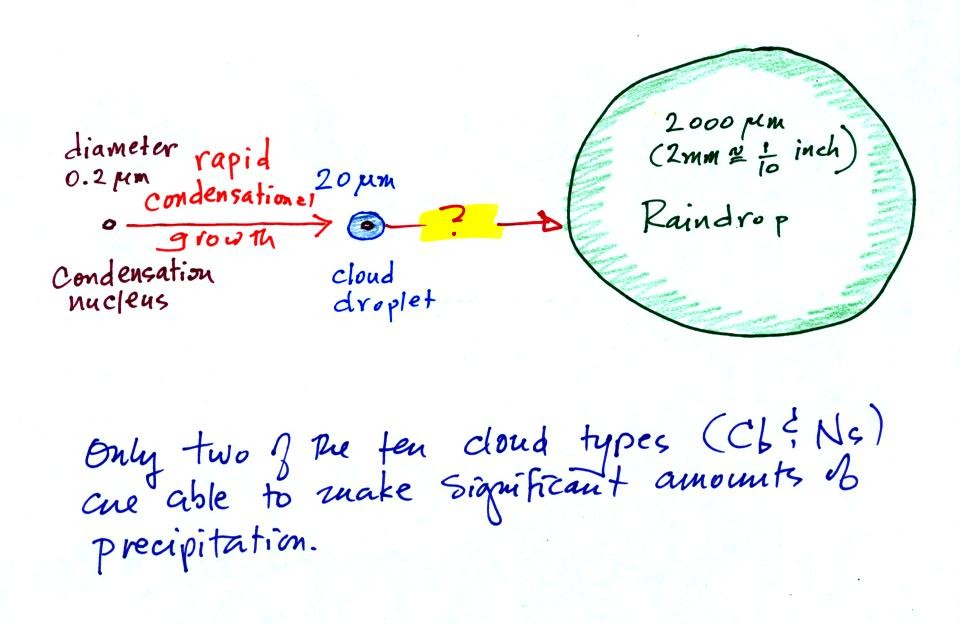
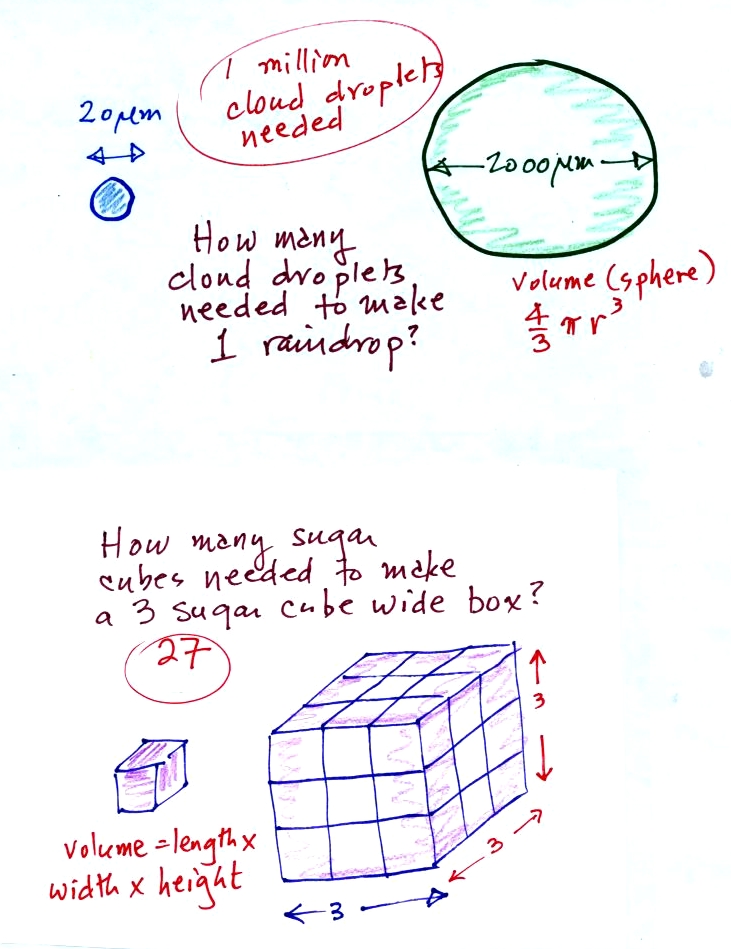
Part of the problem is that it takes quite a few 20 um diameter cloud
droplets to make one 2000 um diameter raindrop. How many
exactly? The raindrop is 100 times bigger across. Volume is
three dimensions. The raindrop is 100 times wider, 100 times
deeper, and 100 times higher than the cloud droplet. The raindrop
has a volume that is 100 x 100 x 100 times larger than the volume of
the cloud droplets.
Fortunately there are two processes capable of quickly
turning small cloud droplets
into much larger precipitation particles in a cloud.

The collision coalescence process works in clouds that are
composed of water droplets only. Clouds like this are only found
in
the tropics. We'll see that this is a pretty easy process to
understand. This process will only produce rain.
The ice crystal process produces precipitation everywhere else.
This is the process that makes rain in
Tucson, even in the hottest part of the summer. There is one part
of this process that is a little harder to understand. Though
this process can produce a variety of different kinds of precipitation
(rain, snow, hail, etc).
Here's
what you might see if you looked inside a warm cloud with just water
droplets:
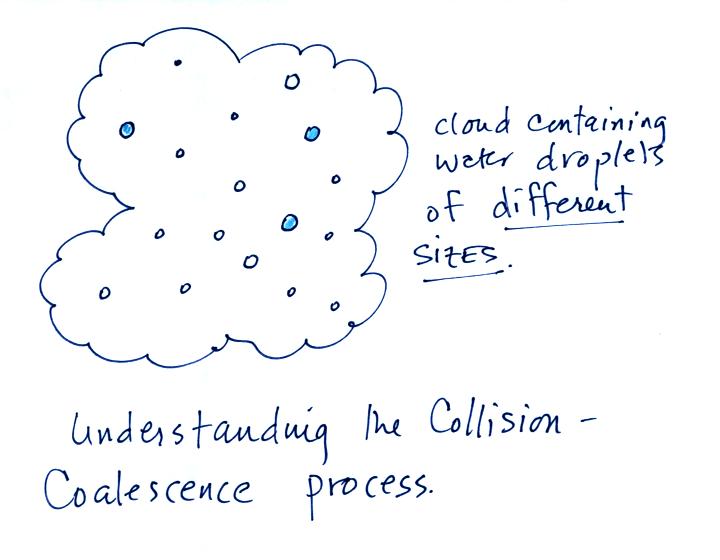
The collision coalescence process works best in a cloud
filled with cloud droplets of different sizes. As we saw in a
short video the larger droplets fall
faster than the small droplets. A larger than average cloud
droplet will overtake and collide with smaller slower moving
ones.

This is an acclerating growth process. The falling droplet
gets
wider, falls faster, and sweeps out an increasingly larger volume
inside the cloud.

The figure
below shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns clouds are thinner
and have weaker updrafts than Cb clouds. Cb clouds are thicker
and have much stronger updrafts. The largest raindrops
fall from Cb clouds because the droplets spend more time in the cloud
growing.
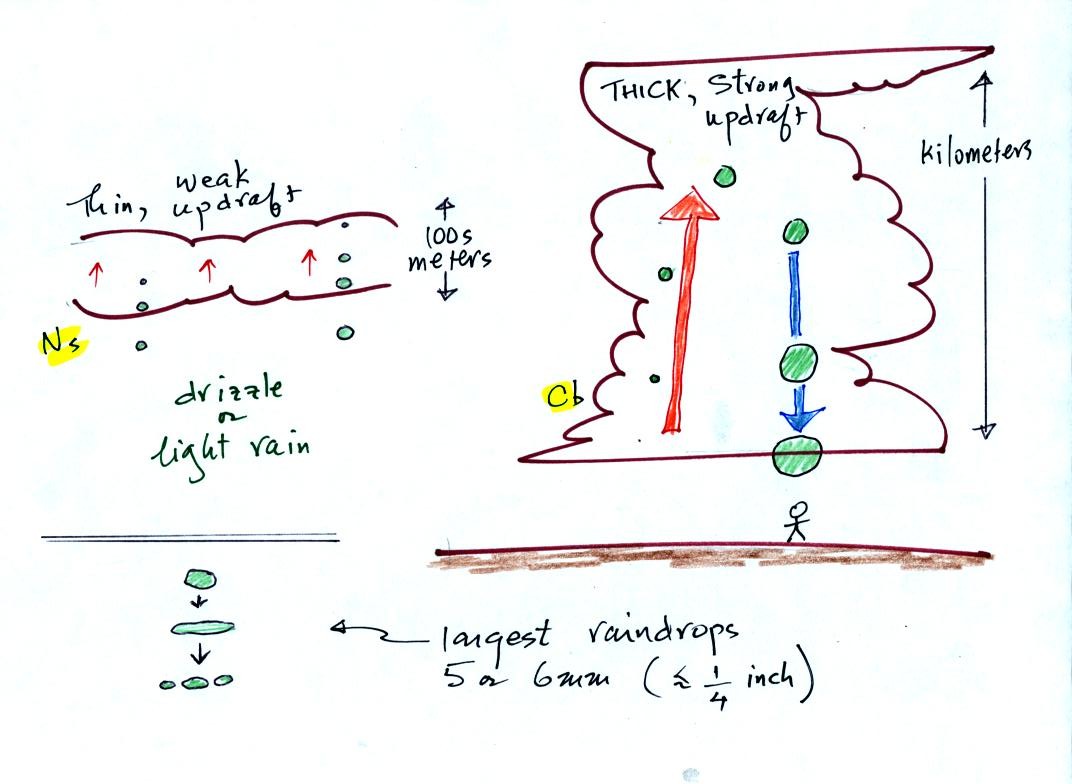
Raindrops grow up to about 1/4 inch in diameter. When
drops get
larger than that, wind resistance flattens out the drop as it falls
toward the ground. The drop begins to "flop" around and breaks
apart
into several smaller droplets. Solid precipitation particles such
as hail can get much larger than 1/4 inch in diameter.
Before
learning about the second precipitation producing process, the ice
crystal process, we need to look at the structure of cold clouds.
The figure below is a redrawn version of what was drawn in class

The bottom of the thunderstorm, Point 1, is warm enough
(warmer than freezing) to just
contain water
droplets. The top of the thunderstorm, Point 2, is colder than
-40 C and just contains ice crystals. The interesting part of the
thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains both
supercooled water
droplets (water that has
been cooled to below freezing but hasn't frozen) and ice
crystals.
This is called the mixed phase
region. This is where the ice crystal process will be able
to produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
The supercooled water droplets aren't able to freeze even though
they
have been cooled below freezing. At Point 4 we see this is
because it is much
easier for small droplets of water to freeze onto an ice crystal
nucleus (just like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small droplet
of pure water). Not just any material will work as an ice nucleus
however. The material must have
a crystalline structure that is like that of ice.
We'll see
next how the ice crystal process works. There's a
couple of tricky parts.
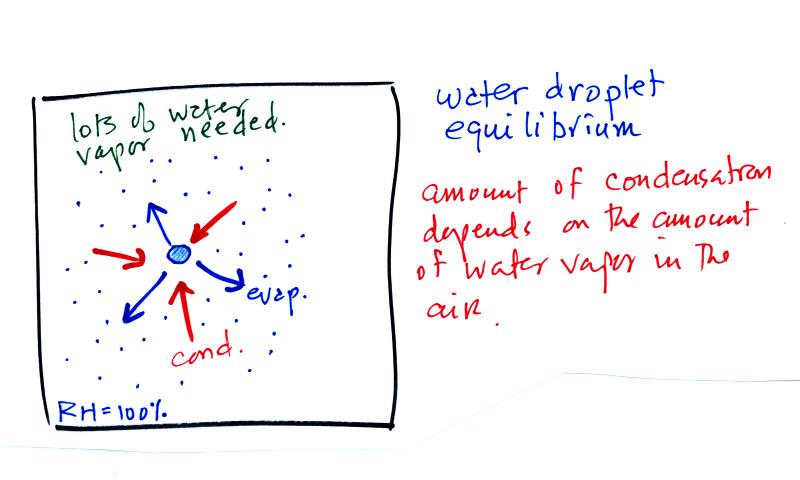
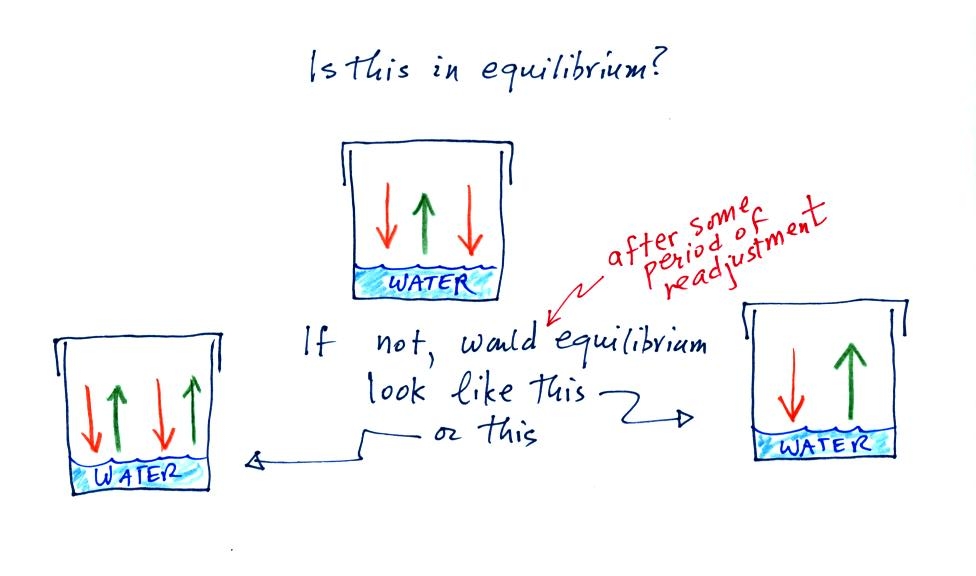
The first figure above (see p.101 in the photocopied Class Notes)
shows a water droplet in equilibrium with its surroundings..The droplet
is evaporating (the 3 blue arrows in the figure). The rate of
evaporation will depend on the temperature of the water droplet.
The droplet is surrounded by air that is saturated with water vapor
(the droplet is inside a cloud where the relative humidity is
100%). This means there is enough water vapor to be able to
supply 3 arrows of condensation.

This figure shows what is required for an ice crystal (at
the same
temperature) to be in equilibrium with its surroundings. First
the ice crystal won't evaporate as rapidly as the water droplet (only
one arrow is shown). Going from ice to water vapor is a bigger
jump than going from water to water vapor. There won't be as many
ice molecules with enough energy to make that jump. A sort of
analogous situation is shown in the figure below. Your fearless
instructor was able to jump from the ground up 20 inches and land on a
chair (a swivelling chair with wheels). But he wasn't willing (he
was pretty he wouldn't be able) to try to jump from the floor up 34
inches and land on the cabinet top near the front of the room.
To be in equilibrium only one arrow of condensation is
needed.
There doesn't need to be as much water vapor in the air surrounding the
ice crystal to supply this lower rate of condensation.
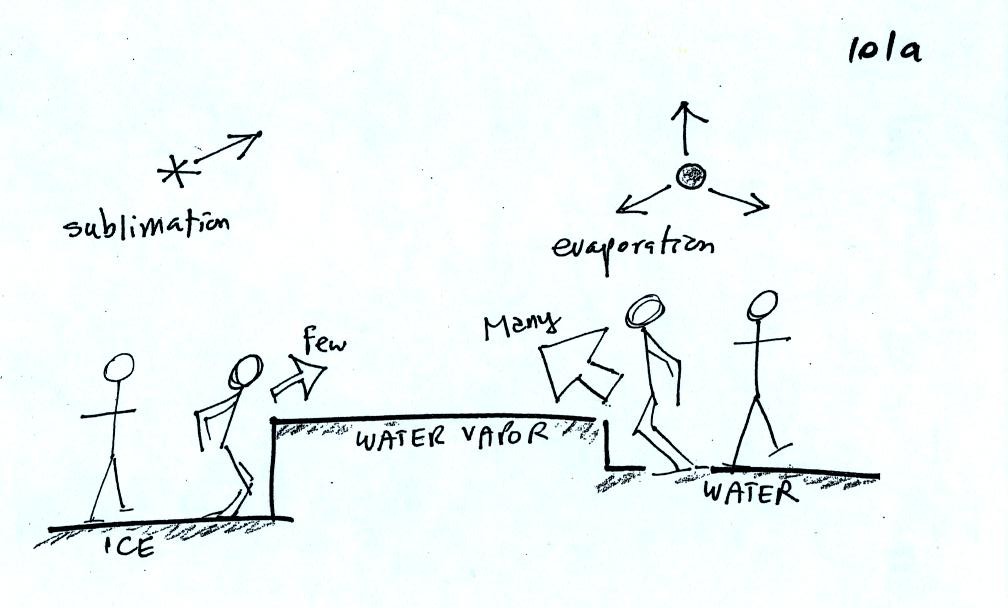
There are going to be fewer people able to make the big jump on
the
left just as there are fewer ice molecules able to sublimate.
Going from water to water vapor is a "smaller jump" and more molecules
are able to do just as more people would be able to make the shorter
jump at right in the picture above.
Now what happens in the mixed phase region of a cold cloud
is that
ice crystals find themselves in the very moist surroundings needed for
water droplet equilibrium. This is shown below.
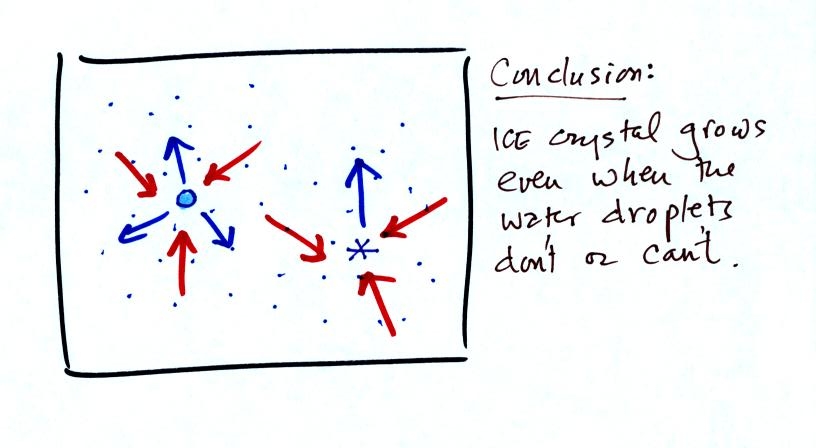
The water droplet is in equilibrium (3 arrows of evaporation
and 3
arrows of condensation) with the surroundings. The ice crystal is
evaporating more slowly than the water droplet. Because the ice
crystal is in the same surroundings as the water droplet water vapor
will be condensing onto the ice crystal at the same rate as onto the
water droplet. The ice crystal isn't in equilibrium, condensation
exceeds evaporation and the ice crystal will grow.
The equal rates of condensation are shown in the figure
below using the
earlier analogy.

Even though he was afraid to jump from the floor to the cabinet top,
the instructor had no problem jumping from the cabinet top to the floor.
Have a
nice weekend, we'll look
at the variety of things that can happen to a growing ice crystal in a
cold cloud in class on Tuesday.




















