
No I wasn't in
defensive driving or traffic school last week. I was in France
last week, in Paris. I had enrolled in a one-week intensive
French course. Here are a
few pictures from Paris.
We reviewed some of the material on dew point and the origin of the atmosphere. This was stuck onto the online notes from Wednesday's class. Briefly most of the gases in today's atmosphere, which is very different from the earth's original atmosphere, are thought to have come from volcanoes. Plants and photosynthesis, not volcanoes, are the source of most of the oxygen in the atmosphere however. Here's what I wrote down while discussing this in class today (this is borrowed from a previous semester and may differ very slightly from what I wrote down in class on Friday).

We returned to the topic of the origin of oxygen and its buildup in the atmosphere at the beginning of today's class. This is summarized on p. 1 in the photocopied ClassNotes.

This somewhat confusing figure shows some of the important events in the history of the earth and evolution of the atmosphere. The numbered points were emphasized.
First, Point 1: the earth is thought to be between 4.5 and 4.6 billion years old.
The iron catastrophe was an important event (but wasn't discussed in class). Circulation of liquid metal in the core of the earth gives the earth a magnetic field. The magnetic field deflects the solar wind around the earth. Remember the solar wind may have swept away the earth's original atmosphere.
Stromatolites (Point 2) are column-shaped structures made up of layers of sedimentary rock, that are created by microorganisms living at the top of the stromatolite (I've never actually seen a stromatolite, so this is all based on photographs and written descriptions). Fossils of the very small microbes (cyanobacteria = blue green algae) have been found in stromatolites as old as 2.7 B years and are some of the earliest records of life on earth. Much older (3.5 to 3.8 B years old) stromatolites presumably also produced by microbes, but without microbe fossils, have been found.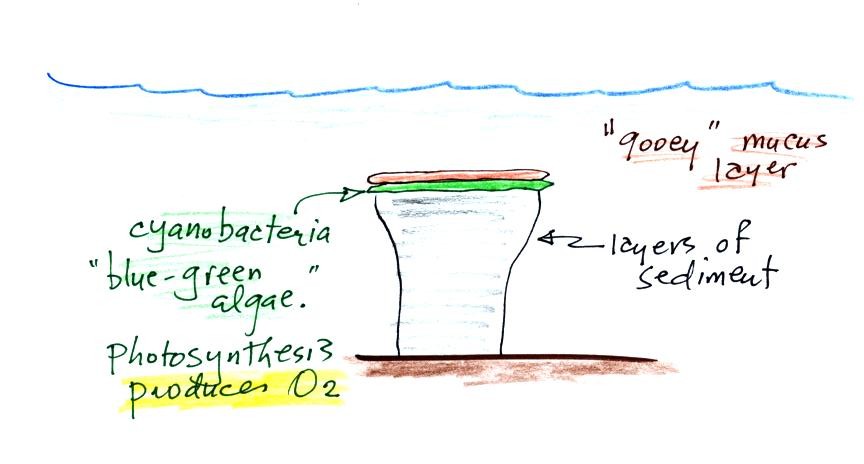

Eventually the dissolved iron in the ocean was used up (Point 4 in the timeline figure above). Oxygen produced by cyanobacteria no longer reacted with iron and was free to diffuse from the ocean into the atmosphere. Once in the air, the oxygen could react with iron in sediments on the earth's surface. This produced red colored (rust colored) sedimentary rock. None of these socalled red beds are older than about 2 B years old. Thus it appears that a real buildup up oxygen began around 2 B years ago. Oxygen concentrations reached levels that are about the same as today around 500 to 600 years ago (Point 5 in the figure).
We listed the 5 most abundant gases in the atmosphere in class on Monday. Several more important trace gases were added to the list in class today. Trace gases are gases found in low concentrations. Low concentrations doesn't mean they aren't important, however.
We reviewed some of the material on dew point and the origin of the atmosphere. This was stuck onto the online notes from Wednesday's class. Briefly most of the gases in today's atmosphere, which is very different from the earth's original atmosphere, are thought to have come from volcanoes. Plants and photosynthesis, not volcanoes, are the source of most of the oxygen in the atmosphere however. Here's what I wrote down while discussing this in class today (this is borrowed from a previous semester and may differ very slightly from what I wrote down in class on Friday).

We returned to the topic of the origin of oxygen and its buildup in the atmosphere at the beginning of today's class. This is summarized on p. 1 in the photocopied ClassNotes.

This somewhat confusing figure shows some of the important events in the history of the earth and evolution of the atmosphere. The numbered points were emphasized.
First, Point 1: the earth is thought to be between 4.5 and 4.6 billion years old.
The iron catastrophe was an important event (but wasn't discussed in class). Circulation of liquid metal in the core of the earth gives the earth a magnetic field. The magnetic field deflects the solar wind around the earth. Remember the solar wind may have swept away the earth's original atmosphere.
Stromatolites (Point 2) are column-shaped structures made up of layers of sedimentary rock, that are created by microorganisms living at the top of the stromatolite (I've never actually seen a stromatolite, so this is all based on photographs and written descriptions). Fossils of the very small microbes (cyanobacteria = blue green algae) have been found in stromatolites as old as 2.7 B years and are some of the earliest records of life on earth. Much older (3.5 to 3.8 B years old) stromatolites presumably also produced by microbes, but without microbe fossils, have been found.

We're learning about stromatolites
because the cyanobacteria were able to produce oxygen using
photosynthesis.

Living stromatolites are found
in a
few locations today. The picture above is from Coral Bay Australia, located on
the
western tip of the continent. The picture was probably taken at
low tide, the stromatolites would normally be covered with ocean water.
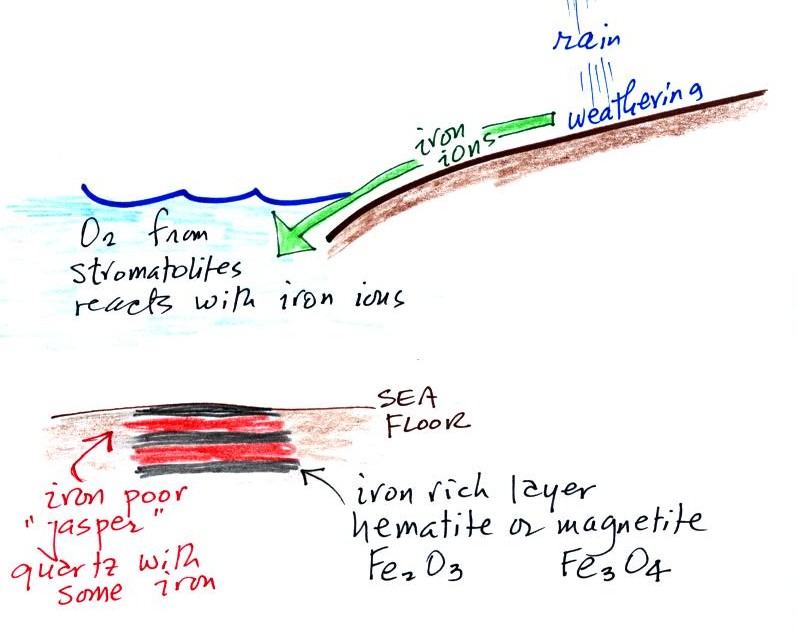
Periodically the oxygen production would decrease or stop (rising oxygen levels might have killed the cyanobacteria or seasonal changes might have slowed the photosynthesus). During these times of low dissolved oxygen concentrations, layers of jasper would form on the ocean bottom. Eventually the cyanobacteria would recover, begin producing oxygen again, and a new layer of hematite or magnetite would form. The rocks that resulted, containing alternating layers of black hematite or magnetite and red layers of jasper are known as the banded iron formation (Point 3). A couple of small polished pieces of banded iron rock (actually "tiger iron") were passed around class (thanks for returning them). In addition to the red and black layers, the tiger iron contains yellow layers made of fibers of quartz. The rocks are fairly heavy because they contain a lot of iron, but the most impressive thing about them in my opinion is their age - they are 2 - 3 billion years old!
Once cyanobacteria began to produce
oxygen in ocean water, the oxygen reacted with dissolved iron (iron
ions in the figure below) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed.
Periodically the oxygen production would decrease or stop (rising oxygen levels might have killed the cyanobacteria or seasonal changes might have slowed the photosynthesus). During these times of low dissolved oxygen concentrations, layers of jasper would form on the ocean bottom. Eventually the cyanobacteria would recover, begin producing oxygen again, and a new layer of hematite or magnetite would form. The rocks that resulted, containing alternating layers of black hematite or magnetite and red layers of jasper are known as the banded iron formation (Point 3). A couple of small polished pieces of banded iron rock (actually "tiger iron") were passed around class (thanks for returning them). In addition to the red and black layers, the tiger iron contains yellow layers made of fibers of quartz. The rocks are fairly heavy because they contain a lot of iron, but the most impressive thing about them in my opinion is their age - they are 2 - 3 billion years old!

Eventually the dissolved iron in the ocean was used up (Point 4 in the timeline figure above). Oxygen produced by cyanobacteria no longer reacted with iron and was free to diffuse from the ocean into the atmosphere. Once in the air, the oxygen could react with iron in sediments on the earth's surface. This produced red colored (rust colored) sedimentary rock. None of these socalled red beds are older than about 2 B years old. Thus it appears that a real buildup up oxygen began around 2 B years ago. Oxygen concentrations reached levels that are about the same as today around 500 to 600 years ago (Point 5 in the figure).
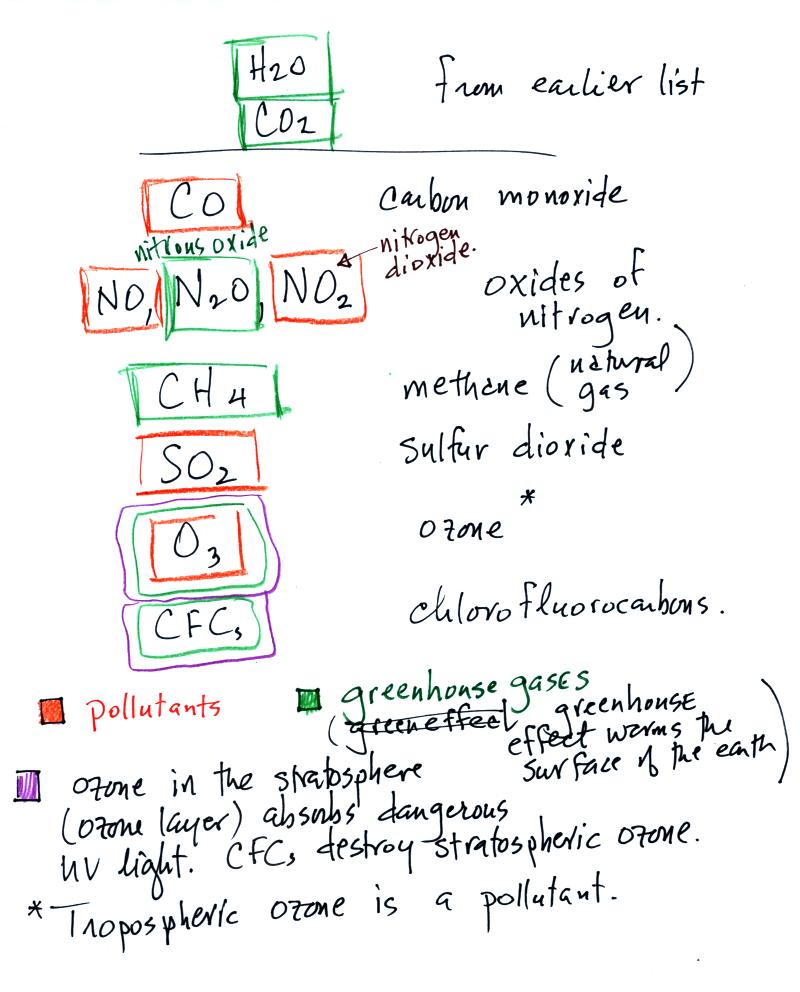
We listed the 5 most abundant gases in the atmosphere in class on Monday. Several more important trace gases were added to the list in class today. Trace gases are gases found in low concentrations. Low concentrations doesn't mean they aren't important, however.

Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing gas),
chlorofluorocarbons, and ozone are all greenhouse gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic in the next week or two and learn more about how the
greenhouse effect actually works later in the course.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur dioxide are some of the major air pollutants. We'll cover these next week.
Be careful with ozone:
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur dioxide are some of the major air pollutants. We'll cover these next week.
Be careful with ozone:
(i) Ozone in the
stratosphere (a layer of the atmosphere between 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is a pollutant and is one of the main ingredients in photochemical smog.
(ii) In the troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is a pollutant and is one of the main ingredients in photochemical smog.
We have
been discussing the composition of air. Air is mostly composed on
invisible gases. I've often thought it might be
interesting to bring in several examples of gases that you can actually
see (the gases are colored, not clear; you can't of course see the
individual gas atoms or molecules). Once I started to do some
research I found that many of these gases are very poisonous. In
some cases a sample large enough for you to be able to see would be a
potentially fatal dose if it were to be released accidentally into the
classroom. You're going to have to settle for pictures of chlorine (a gas with a
yellow-green color), and bromine
(a liquid that evaporates, the resulting gas has a very vivid reddish
color). The caution on the www.webelements.com website: "Bromine
is a serious health hazard and maximum safety precautions should be
taken when handling it" worried me a little bit. I brought in
some iodine (a solid
that sublimates producing a gas with a faint pink color) and showed it
on the Elmo (it wasn't too impressive, the faint pink color was hard to
see). Iodine is poisonous but not nearly as scary as some of
these other
gases.
We did make some nitrogen dioxide, a toxic pollutant. We did this by putting an ordinary copper penny (Cu(s) in the equation below) into a large 4 liter glass flask that contained a small amount of concentrated nitric acid ( HNO3(aq) ).
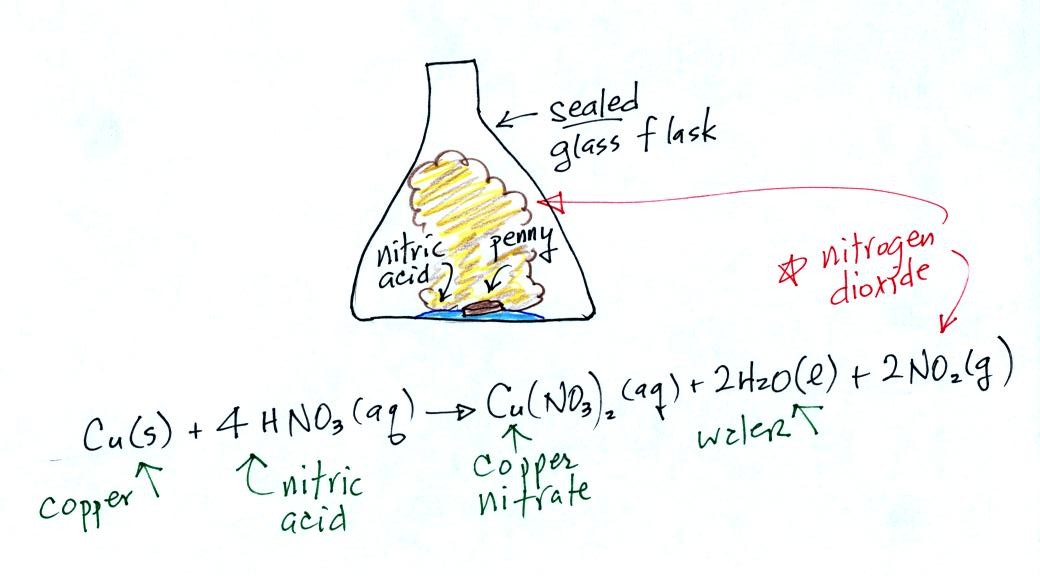
We did make some nitrogen dioxide, a toxic pollutant. We did this by putting an ordinary copper penny (Cu(s) in the equation below) into a large 4 liter glass flask that contained a small amount of concentrated nitric acid ( HNO3(aq) ).

Not a particularly
educational demonstration.
We'll be discussing air pollution in class next week. Air Pollution is a serious health hazard in the US and around the world (the NO2 demonstration maybe reinforces that fact). The following statistics weren't shown in class. Click here to download a copy of this handout. We'll go over this quickly at the start of class next Wednesday.
We'll be discussing air pollution in class next week. Air Pollution is a serious health hazard in the US and around the world (the NO2 demonstration maybe reinforces that fact). The following statistics weren't shown in class. Click here to download a copy of this handout. We'll go over this quickly at the start of class next Wednesday.